Free alkali crystal form of phenylpropionamide derivative and preparation method of free alkali crystal form
A technology of crystal form and diffraction angle, which is applied in the preparation methods of peptides, chemical instruments and methods, and medical preparations containing active ingredients, etc., can solve the problems of poor fluidity, high hygroscopicity, and poor product stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
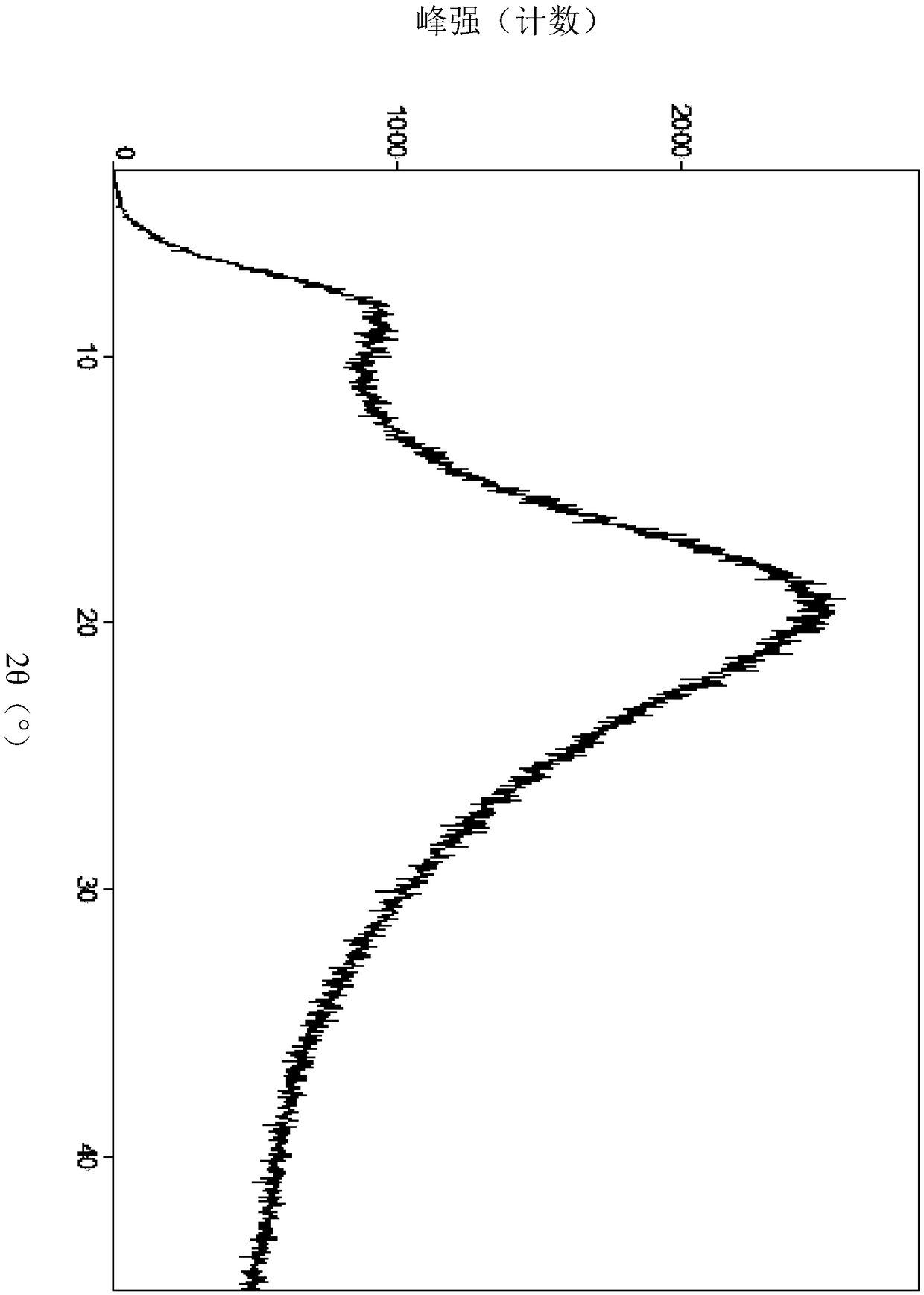
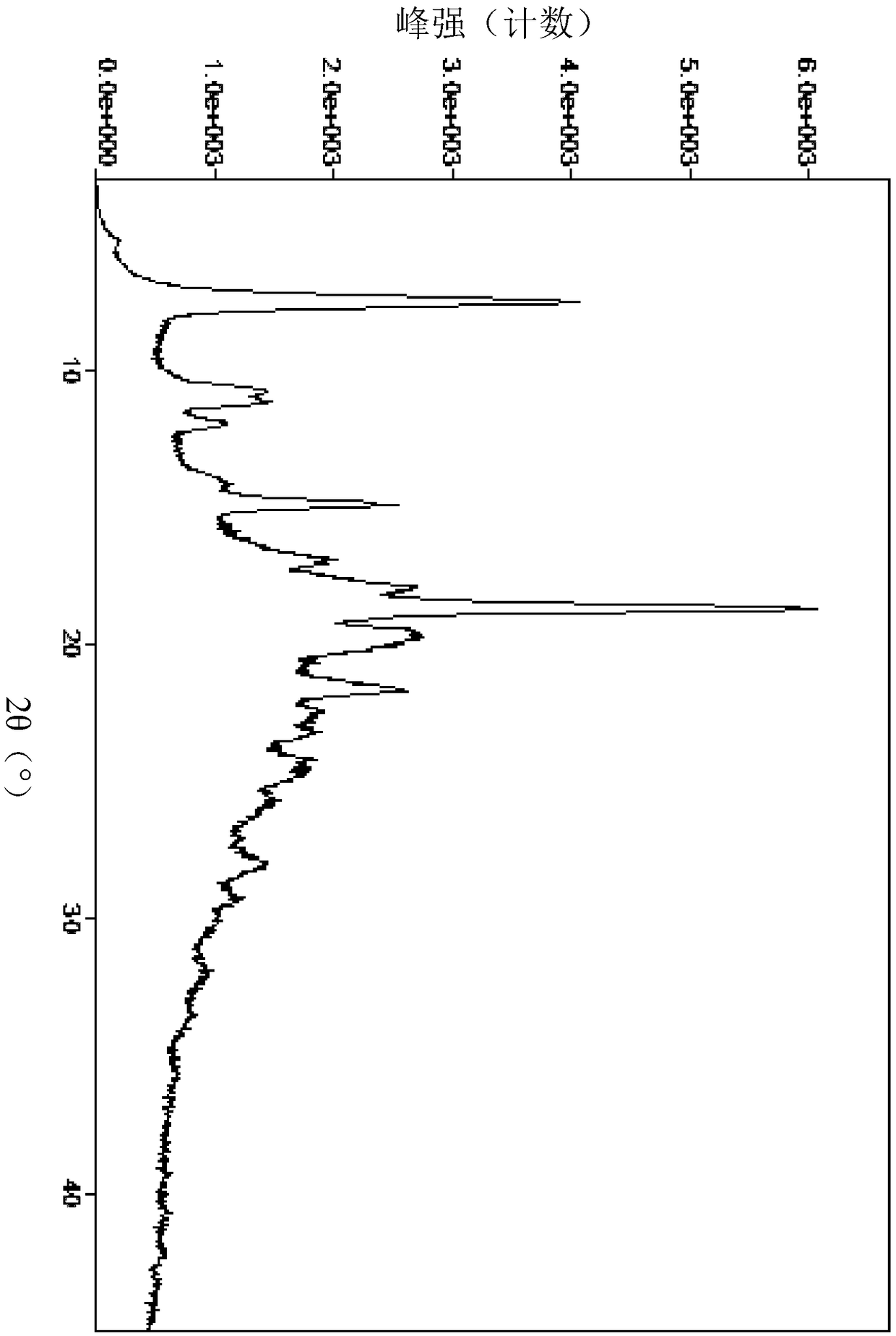
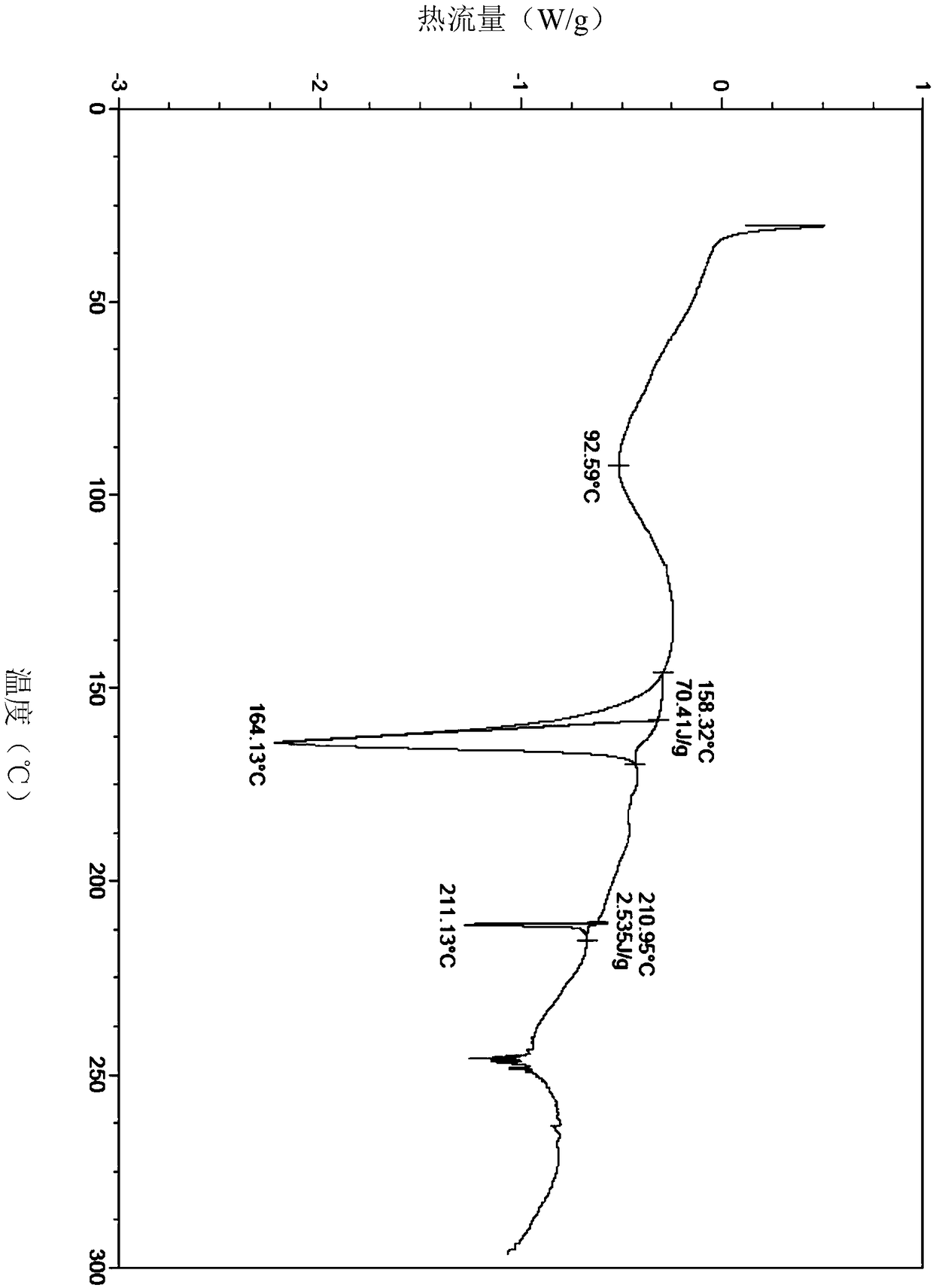
Image
Examples
Embodiment Construction
[0018] In the specification and claims of the present application, unless otherwise specified, the scientific and technical terms used herein have the meanings commonly understood by those skilled in the art. However, for a better understanding of the present invention, definitions and explanations of some related terms are provided below. In addition, when the definitions and explanations of terms provided in this application are inconsistent with the meanings commonly understood by those skilled in the art, the definitions and explanations of terms provided in this application shall prevail.
[0019] The "C1-6 alkyl" in the present invention means a linear or branched alkyl group containing 1-6 carbon atoms. Specific examples include but are not limited to: methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, n-pentyl, isopentyl, 2-methylbutyl, neopentyl, 1-ethylpropyl, n-hexyl, isohexyl, 3- Methylpentyl, 2-methylpentyl, 1-methylpentyl, 3,3-dimethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com