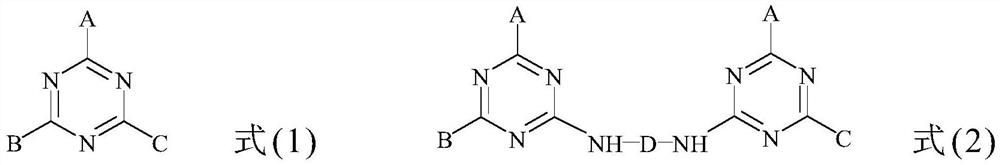
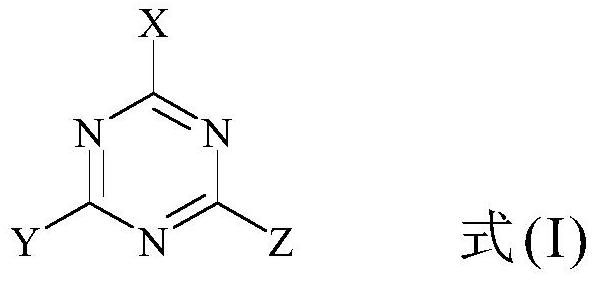
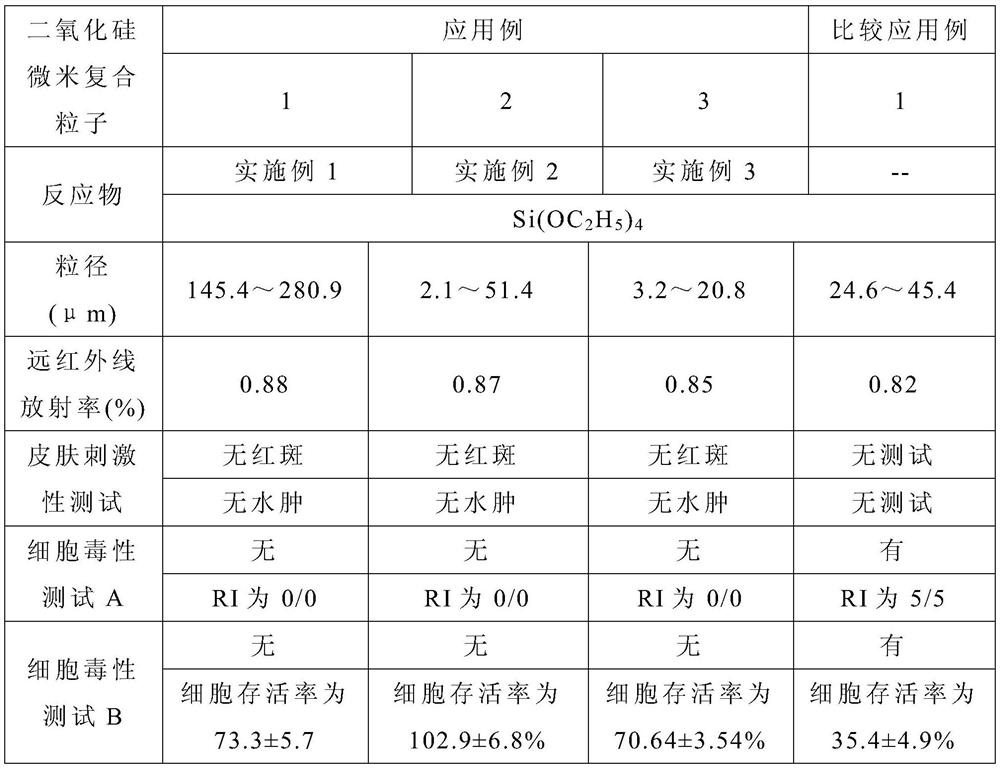
Triazine precursor, method for preparing triazine precursor and application of triazine precursor
A technology of triazine series and precursors, which is applied in the field of preparing the triazine series precursors, the application of the triazine series precursors, triazine series precursors containing siloxane groups, and can solve the problem of silica nanometer Poor particle yield, poor storage stability, self-aggregation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 (C 8 h 17 O)((C 8 h 17 ) 2 N)-Tz-Si(OEt) 3
[0035] Step a: Place a 100 mL single-necked reaction flask containing 30 mL of dry dichloromethane in an ice-water bath at 0°C. 1.84 grams (10 mmol) of cyanuric chloride was added to the reaction flask, and the cyanuric chloride was dissolved. Then, 2.41 g (10 mmol) of dioctylamine was slowly dropped into the reaction flask. After reacting for 10 minutes, 1.01 g (10 mmol) of triethylamine was slowly added. After reacting for 20 minutes, 30 mL of 0.5 M aqueous sodium hydroxide solution was used for extraction twice, and the organic layer was collected. Add 50 mL of clear water to the organic layer for extraction treatment, and collect the organic layer again. Next, adding anhydrous magnesium sulfate to the organic layer to remove water, and then filtering to obtain a filtrate. Dichloromethane in the filtrate was removed using a vacuum concentrator to obtain 3.69 g (9.5 mmol) of 2,4-dichloro-6-dioctylamin...
Embodiment 2
[0039] Embodiment 2 (C 2 h 5 O)((C 8 h 17 ) 2 N)-Tz-Si(OEt) 3
[0040] The difference between this Example 2 and this Example 1 is that the 1-octanol in step b of Example 1 is replaced by ethanol, and the compound formed after step b is completed is 2-chloro-4-dioctylamino -6-Ethyloxy-1,3,5-triazine. And replace 2-chloro-4-dioctylamino-6-octyloxy-1,3,5-triazine in step c with 2-chloro-4-dioctylamino-6-ethane oxy-1,3,5-triazine, and the compound formed after completion of step c is 2-dioctylamino-4-ethyloxy-6-(3-triethoxysilylpropyl ) amino-1,3,5-triazine. The overall yield for this Example 2 was 47.0%.
[0041] Spectral analysis of the 2-dioctylamino-4-ethyloxy-6-(3-triethoxysilylpropyl)amino-1,3,5-triazine: 1 H NMR (300MHz, CDCl 3 , δ(ppm): 0.62(t, J=8.1Hz, 2H, CH 2 -Si), 0.86(t, J=6.3Hz, 6H, 2×CH 3 ), 1.20(t, J=6.9Hz, 9H, 3×CH 3 -COSi), 1.27(s, broad, 23H, 10×CH 2 +CH 3 ),1.55(s,broad,4H,2×CH 2 -CN), 1.60~1.78(m,2H,CH 2 -CSi),3.33~3.51(m,6H,3×CH 2 -N),3.80...
Embodiment 3
[0042] Embodiment 3 ((CH 3 ) 2 CHO)((C 8 h 17 ) 2 N)-Tz-Si(OEt) 3
[0043] The difference between this Example 3 and this Example 1 is that the 1-octanol in step b of Example 1 is replaced with isopropanol, and the compound formed after step b is completed is 2-chloro-4-dioctyl Amino-6-isopropyloxy-1,3,5-triazine. And replace 2-chloro-4-dioctylamino-6-octyloxy-1,3,5-triazine in step c with 2-chloro-4-dioctylamino-6-iso Propyloxy-1,3,5-triazine, while the compound formed after completion of step c is 2-dioctylamino-4-isopropyloxy-6-(3-triethoxysilyl Propyl)amino-1,3,5-triazine. The overall yield for this Example 3 was 38.3%.
[0044] Spectral analysis of the 2-dioctylamino-4-isopropyloxy-6-(3-triethoxysilylpropyl)amino-1,3,5-triazine: 1 H NMR (300MHz, CDCl 3 ), δ (ppm): 0.61 (t, J = 8.1Hz, 2H, CH 2 -Si), 0.85(t, J=6.3Hz, 6H, 2×CH 3 ), 1.19(t, J=6.9Hz, 9H, 3×CH 3 -COSi), 1.31(s, broad, 26H, 13×CH 2 ),1.55(s,broad,4H,2×CH 2 -CN), 1.69(m, J=6.6Hz, 2H, CH 2 -CSi), ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com