Synthetic method of pholcodine
A synthetic method and pholkidine technology, applied in organic chemistry methods, organic chemistry and other directions, can solve the problems of large required amount, many reaction steps, reduced difficulty and safety risk, etc., and achieve easy quality and simple reaction steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
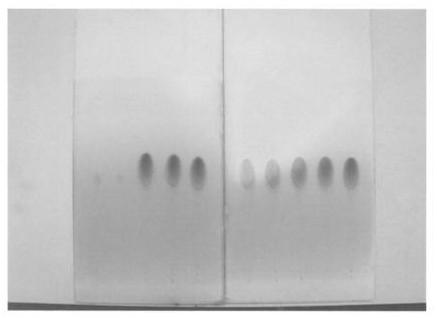
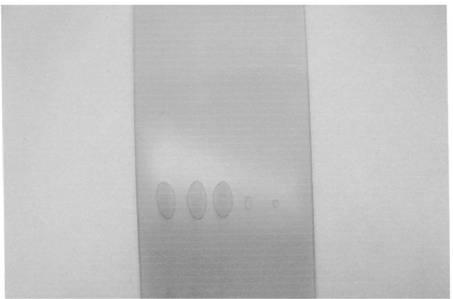
Image
Examples
Embodiment 1
[0048] 1) 285.3g morphine base is dissolved in the DMF solution of 2000ml.
[0049] 2) Dissolve 282g of 1,2-dibromoethane in 800ml of DMF solution and add 110g of anhydrous sodium carbonate. Under nitrogen protection, the temperature was raised to 60° C., and the DMF solution of morphine base prepared in 1) was slowly dropped into the 1,2-dibromoethane solution under stirring. After the dropwise addition was completed, the insulation reaction was continued for 2 hours.
[0050] 3) After the reaction is complete, distill under reduced pressure until there is no liquid drop, add 550ml of purified water and stir to dissolve the inorganic salt. The insoluble matter was collected by filtration and dried.
[0051] 4) 105g morpholine and 3) insolubles are dissolved in 2000ml DMF, and add 110g anhydrous sodium carbonate and 10g nano CuI powder. Under the protection of nitrogen, the temperature was raised to 115° C. for 6 hours.
[0052] 5) Suction filtration while hot, collect the...
Embodiment 2
[0064] 1) 285.3g morphine base is dissolved in the DMF solution of 2000ml.
[0065] 2) Dissolve 423g of 1,2-diiodoethane in 800ml of DMF solution and add 110g of anhydrous sodium carbonate. Under nitrogen protection, the temperature was raised to 60° C., and the DMF solution of morphine base prepared in 1) was slowly dropped into the 1,2-diiodoethane solution under stirring. After the dropwise addition was completed, the insulation reaction was continued for 2 hours.
[0066] 3) After the reaction is complete, distill under reduced pressure until there is no liquid drop, add 550ml of purified water and stir to dissolve the inorganic salt. The insoluble matter was collected by filtration and dried.
[0067] 4) 105g morpholine and 3) insolubles are dissolved in 2000ml DMF, and add 110g anhydrous sodium carbonate and 10g nano CuI powder. Under the protection of nitrogen, the temperature was raised to 115° C. for 8 hours.
[0068] 5) Suction filtration while it is hot, collect...
Embodiment 3
[0078] 1) Dissolve 285.3g of morphine base in 2000ml of DMSO solution.
[0079] 2) Dissolve 282g of 1,2-dibromoethane in 800ml of DMSO solution and add 140g of anhydrous potassium carbonate. Under nitrogen protection, the temperature was raised to 60° C., and the DMF solution of morphine base prepared in 1) was slowly dropped into the 1,2-dibromoethane solution under stirring. After the dropwise addition was completed, the insulation reaction was continued for 2 hours.
[0080] 3) After the reaction is complete, distill under reduced pressure until there is no liquid drop, add 550ml of purified water and stir to dissolve the inorganic salt. The insoluble matter was collected by filtration and dried.
[0081] 4) 105g morpholine and 3) insolubles are dissolved in 2000ml DMSO, and 140g anhydrous potassium carbonate and 10g nano CuI powder are added. Under the protection of nitrogen, the temperature was raised to 120° C. for 7 hours.
[0082] 5) Suction filtration while hot, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com