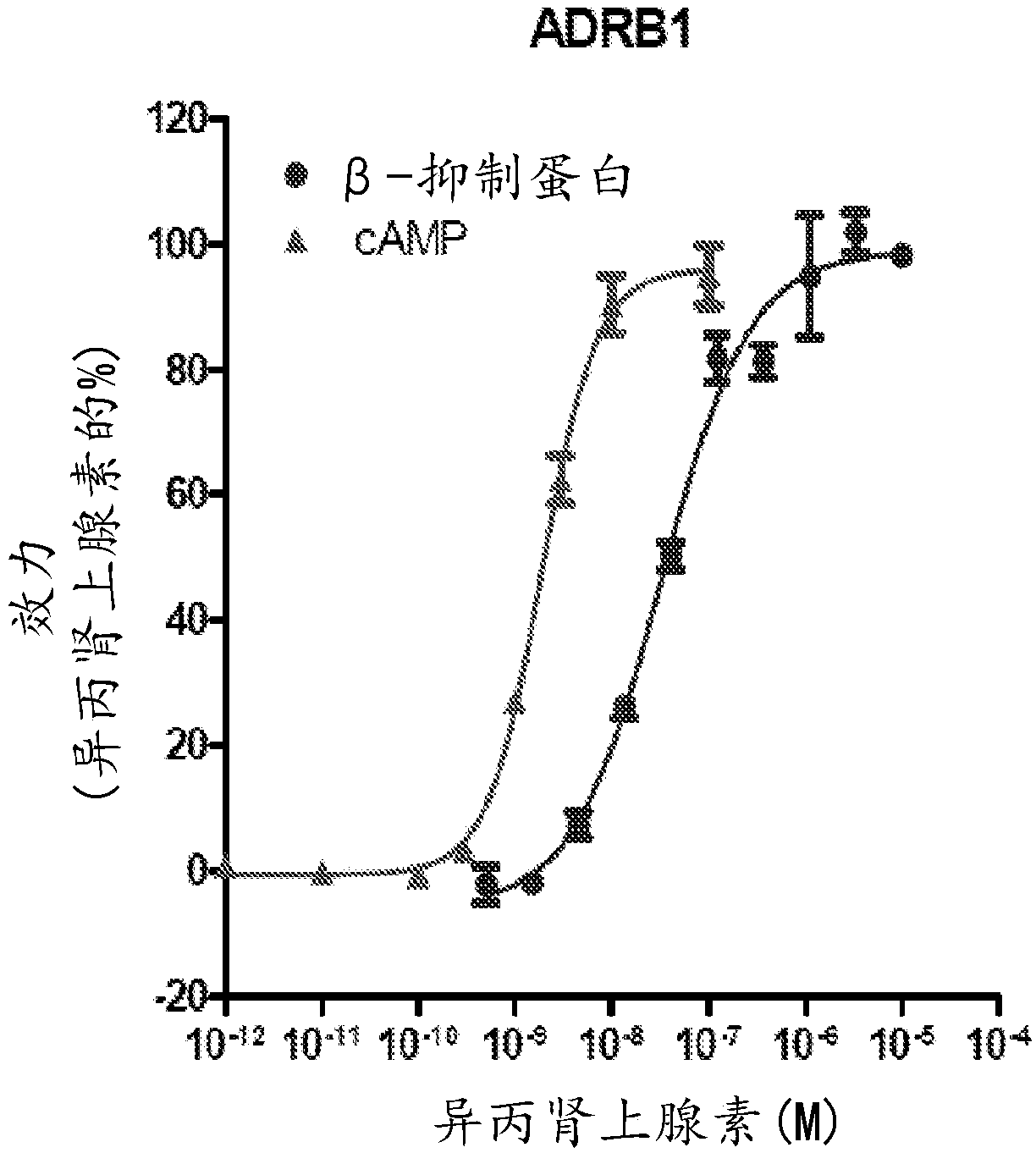
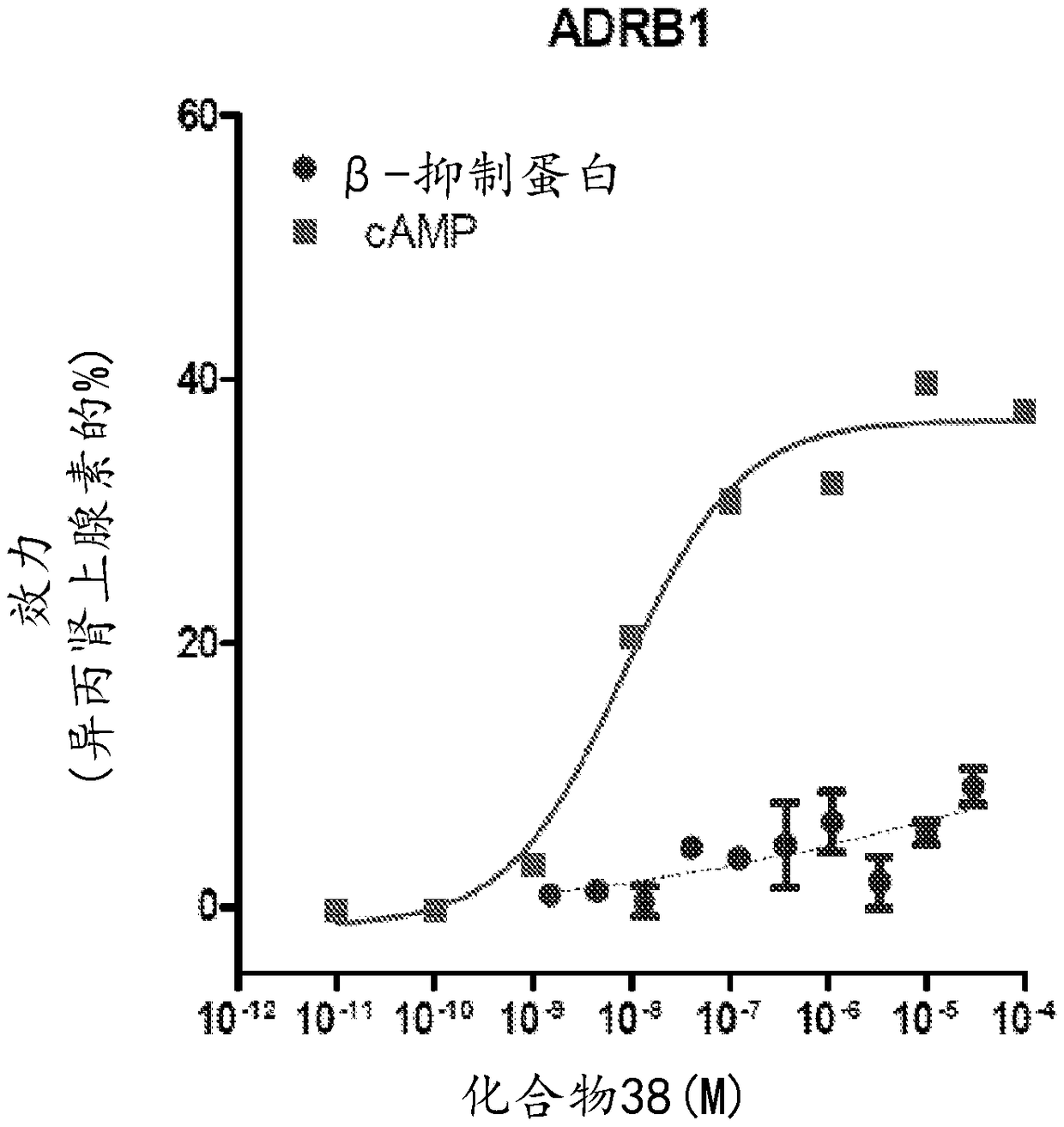
Adrenergic receptor modulating compounds and methods of using same
A technology of adrenaline and compounds, applied in the field of adrenergic receptor modulating compounds and their use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0400] Example 1: Exemplary synthesis of compounds The following representative synthetic methods and strategies can be applied to prepare the subject compounds.
[0401] plan 1:
[0402]
[0403] Reagents and conditions: a, hydrazine hydrate, ethylene glycol, 25-160℃, 2.5 hours; B, (R)-epichlorohydrin, CSF, DMF, 50℃, 48 hours; C, 4,2-propanol , 50°C, 12 hours.
[0404] 3-Methyl-1H-indazol-4-ol (2): The 1-(2,6-dihydroxyphenyl)ethan-1-one (1) (5.0g, 32.86mmol) in ethylene glycol ( 70ml) was added to a solution of hydrazine hydrate (3.29g, 65.72mmol) in ethylene glycol (20mL). The reaction mixture was stirred at room temperature for 20 minutes and then heated at 160°C for an additional 2 hours. After cooling to room temperature and diluting with water (200 mL), HOAc (2.5 ml) was added to adjust pH=6. The resulting mixture was extracted with EtOAc (3×50 ml). The organic layer was washed with brine and subjected to Na 2 SO 4 Dry, filter, and concentrate the filtrate under reduced ...
Embodiment 2
[0451] Example 2: Synthesis of compound
[0452] Reaction scheme: Step 1
[0453] Process: To a mixture of 1-(2,6-dihydroxyphenyl)ethanone (10g, 65.7mmol) in ethylene glycol (140mL) was added hydrazine hydrate (6.58g, 131.4mmol) in ethylene glycol (40mL ) Solution. The reaction mixture was stirred at room temperature for 20 minutes and at 160°C for an additional 2 hours. After cooling to ambient temperature, the RM was diluted with water (400 mL). Acetic acid (5 mL) was added to adjust pH=6. The resulting mixture was extracted with EtOAc (3×100 mL). The organic layer was washed with brine and subjected to Na 2 SO 4 It was dried, filtered, concentrated under reduced pressure, and purified by crystallization (9.9 g, 100%) as a brown solid.
[0454] Reaction scheme: Step 2
[0455]
[0456] Process: To the product of step-1 (2g, 13.5mmol), TEA (5.61mL, 40.4mmol) and DMAP (100mg) in anhydrous THF (35mL) was added dropwise (BOC) at -15°C 2 A solution of O (2.94 g, 13.5 mmol) in THF (...
Embodiment 3
[0545] Example 3: Assay for evaluating compounds
[0546] Assays that may be suitable for assessing the activity of the subject compound include those described by Shamloo et al., Neurobiology of Disease 43 (2011) 397-413. In addition, the following materials and general methods can be adapted to assess the activity of the subject compound, for example, the modulation and pathology and activity of neuroinflammation in Alzheimer's disease (AD) models.
[0547] Behavior test
[0548] Open field: Under low light conditions, evaluate general sports activities in a square field (76cm×76cm×50cm). The mouse was placed in a corner of the open field and allowed to explore freely for 10 minutes while being tracked from a camera installed on the ceiling by an automatic tracking system (Ethovision). At the end of each test, the surface of the field was cleaned with 1% Virkon disinfectant.
[0549] Activity Room : Evaluate general motor activity as previously described (Shamloo et al., (2011)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com