Application of a gold nanoparticle modified by a ferrocene amide molecular compound in pharmacy
A molecular compound and gold nanoparticle technology, which is applied to surface functionalized nanomaterials and their application fields to achieve the effect of diversifying redox catalytic activities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
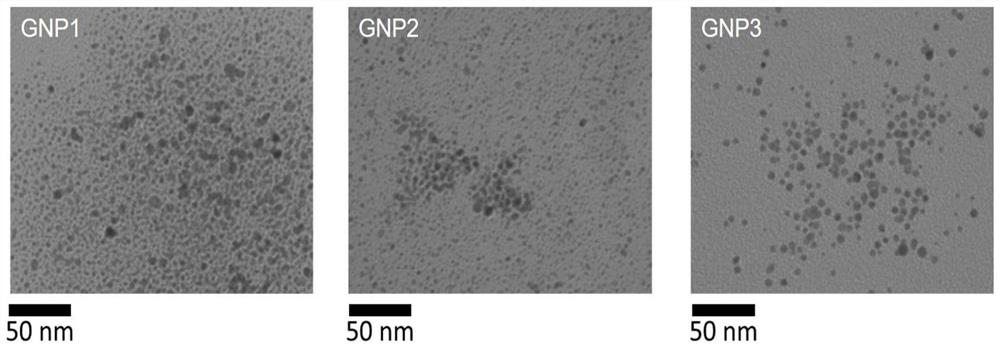
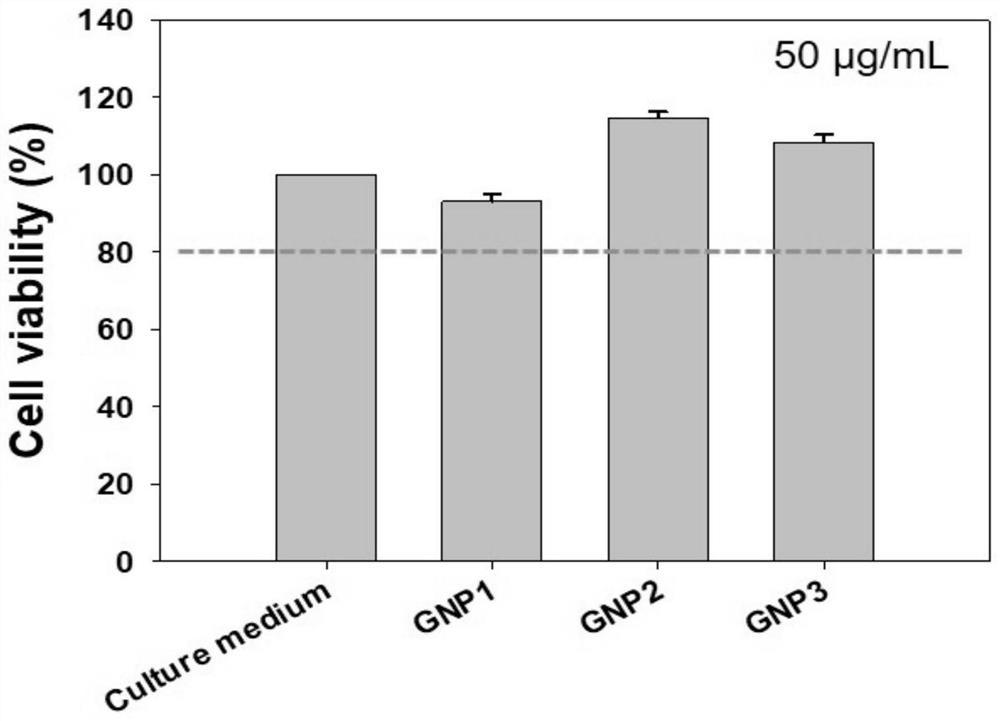
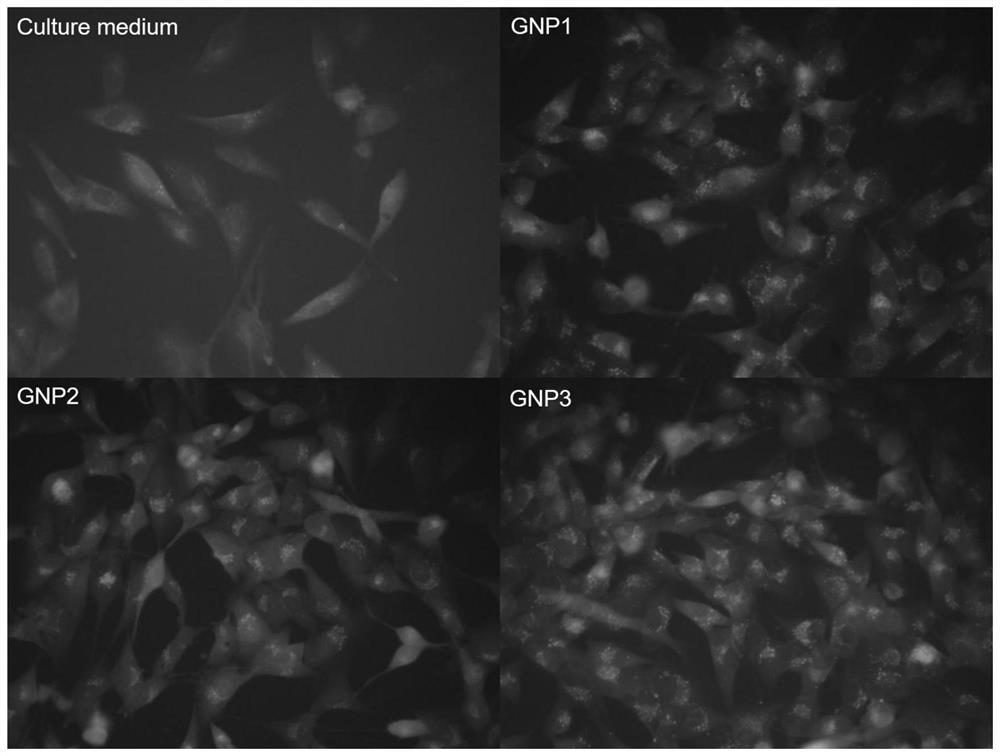
[0047] Example 1 Taking 3,6-dioxoctanediamine and gold nanoparticles (GNP1) modified by benzaldehyde ferrocene derivative molecular compound (Ligand1) as an example to illustrate the modification of ferrocene amide molecular compound described in the present invention Synthesis of gold nanoparticles
[0048] a. Under an ice bath, mix ferrocenecarboxylic acid (11.5 g, 0.05 mol) with 100 mL of dichloromethane (DCM), and stir evenly. Under vigorous stirring, N-hydroxysuccinimide (NHS) (7.0 g, 0.06 mol), 1-(3-dimethylaminopropyl)-3-ethyl carbon Diimine hydrochloride (EDC·HCl) (11.5 g, 0.06 mol). After reacting in an ice bath for 4-6 hours, the solution gradually became clear, and the reaction was monitored by TLC.
[0049] After the reaction was completed, it was suction filtered to obtain a dichloromethane solution of the intermediate.
[0050] b. Under an ice bath, (8.5 g, 0.075 mol) triethylenediamine and 50 mL of dichloromethane (DCM) were mixed and dissolved and stirred ev...
Embodiment 2
[0053] Example 2 Synthesis of gold nanoparticles (GNP2) modified by p-phenylenediamine and benzaldehyde ferrocene derivative molecular compound (Ligand2)
[0054] The preparation method is similar to the preparation of GNP1 in Example 1, the difference is that 32.6mL of 3,6-dioxoctanediamine used in step b is replaced by 27.5mL of p-phenylenediamine, and finally the compound Ligand2 is obtained as a light yellow powder ; The 0.055 g ligand compound Ligand1 used in step d was replaced by 0.056 g ligand compound Ligand2.
[0055] The reaction pathway is shown in formula (IV):
[0056]
Embodiment 3
[0057] Example 3 Synthesis of gold nanoparticles (GNP3) modified by p-phenylenediamine, 4-N, N-dimethylaminobenzaldehyde ferrocene derivative molecular compound (Ligand3)
[0058] The preparation method is similar to the preparation in Example 1, the difference is that the 0.50mL benzaldehyde used in step c is replaced by 0.64mL 4-N,N-dimethylaminobenzaldehyde, and finally the compound Ligand3 is obtained as a light yellow powder ; The 0.055 g ligand compound Ligand1 used in step d was replaced by 0.060 g ligand compound Ligand3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com