Analysis method of chiral isomer
An isomer and volume technology, applied in the field of analysis of pharmaceutical raw materials, can solve the problems of not mentioning the separation of diastereomers, not being able to detect three isomers at the same time, and not realizing baseline separation, etc. Ease of standardization, method specificity and reproducibility, and good separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Resolution solution: A mixed solution containing palonosetron hydrochloride and 3 isomers was prepared with ethanol, with a concentration of 0.25 mg / ml, respectively.
[0040] HPLC operating conditions: chiral column (DAICEL CHIRALPAK AD-H 250×4.6mm, 5μm) with tris(3,5-dimethylphenylcarbamate) amylose as filler, n-hexane-ethanol -Methanol-trifluoroacetic acid-diethylamine (volume ratio=75:20:5:0.2:0.1) as mobile phase; flow rate is 1ml / min; column temperature is 35℃; detection wavelength is 240nm; injection volume is 5μL .
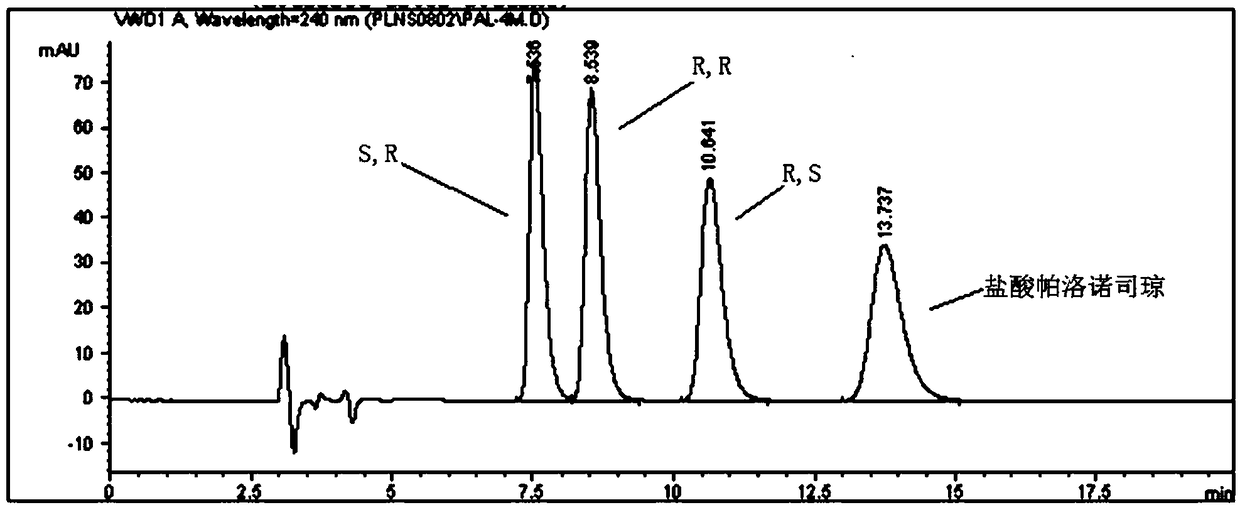
[0041] Take the resolution solution and inject it into the liquid chromatograph to get the attached solution. figure 1 chromatogram shown. S, R, R, R, R, S isomers and palonosetron hydrochloride appeared successively, and the retention times were 7.5 min, 8.5 min, 10.6 min and 13.7 min, respectively.
Embodiment 2
[0043]Resolution solution: A mixed solution containing palonosetron hydrochloride and 3 isomers was prepared with ethanol, with a concentration of 0.25 mg / ml, respectively.
[0044] HPLC operating conditions: chiral column (DAICEL CHIRALPAK AD-H 250×4.6mm, 5μm) with tris(3,5-dimethylphenylcarbamate) amylose as filler, n-hexane-ethanol -Methanol-trifluoroacetic acid-diethylamine (volume ratio=70:25:5:0.2:0.1) as mobile phase; flow rate is 1ml / min; column temperature is 35℃; detection wavelength is 240nm; injection volume is 5μL .
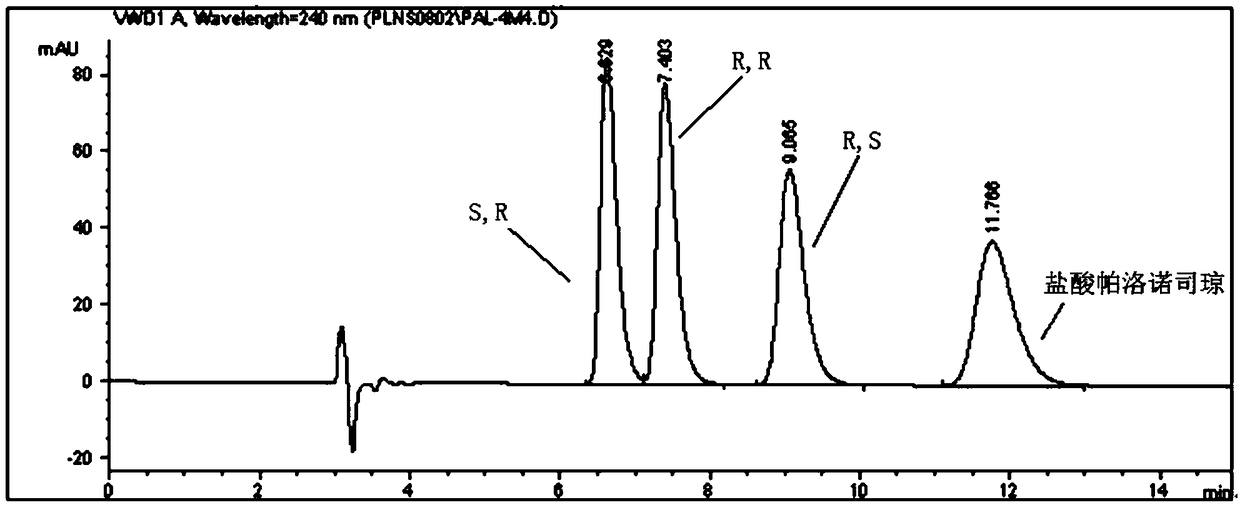
[0045] Take the resolution solution and inject it into the liquid chromatograph to get the attached solution. figure 2 chromatogram shown. S, R, R, R, R, S isomers and palonosetron hydrochloride appeared successively, and the retention times were 6.8 min, 7.4 min, 9.1 min and 11.8 min, respectively.
Embodiment 3
[0047] Control solution: Precisely weigh appropriate amount of Palonosetron hydrochloride and 3 isomer reference substances, dissolve in methanol, and prepare 0.5 μg / ml with diluent (methanol-mobile phase (volume ratio = 1:9)). mixture.
[0048] Test solution: take an appropriate amount of palonosetron hydrochloride, and prepare a solution of 5 mg / ml with diluent (methanol-mobile phase (volume ratio=1:9)).
[0049] HPLC operating conditions: chiral column (DAICEL CHIRALPAK AD-H 250×4.6mm, 5μm) with tris(3,5-dimethylphenylcarbamate) amylose as filler, n-hexane-ethanol -Methanol-trifluoroacetic acid-diethylamine (volume ratio=70:25:5:0.2:0.1) as mobile phase; flow rate is 1ml / min; column temperature is 40°C; detection wavelength is 240nm; injection volume is 20μL .
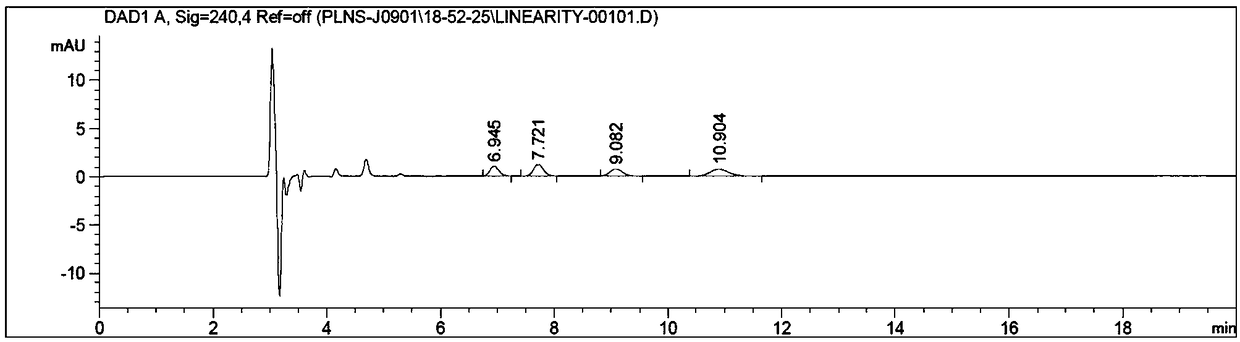
[0050] Take the control solution and the test solution and inject them into the liquid chromatograph, respectively, and obtain the attached image 3 and attached Figure 4 chromatogram shown. S,R isomer, R,R is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com