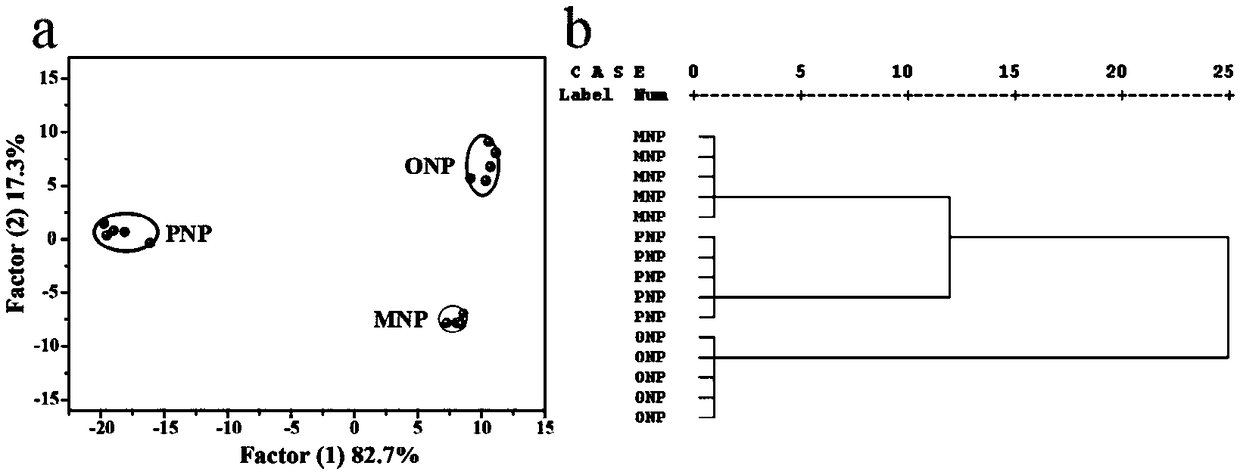
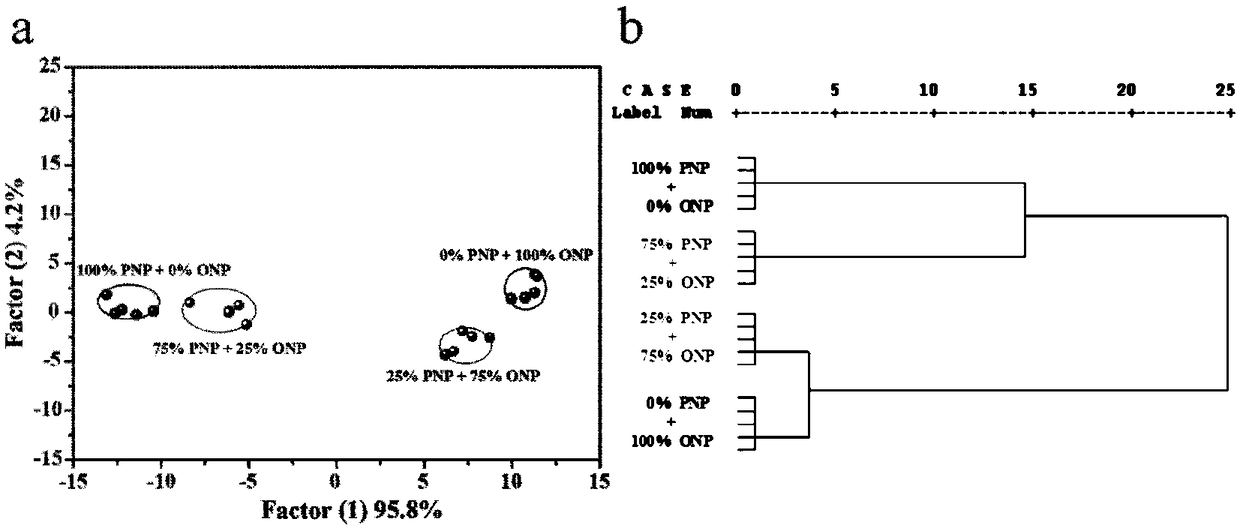
Nitrophenol isomer detection array
A technology of nitrophenol and detection array, which is applied in the field of chemical analysis, can solve the problem of not being able to distinguish o-nitrophenol well, and achieve the effect of good discrimination and good dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Synthesis of β-CD and mercaptoundecanoic acid (MUA) bifunctional gold nanoclusters (Au NC1): under stirring, first mix 100 μL NaOH (1M) solution and 24 μL THPC (8%, wt) solution with 8 mL Mix with ultrapure water for 5 min, then add 96 μL HAuCl 4 (Au 3+ , 25mM) solution. Then, 100 μL of β-CD-SH (10 mM) solution was added to obtain β-CD protected gold nanoparticles (β-CD-Au NPs). After stirring at room temperature for an additional 15 minutes, the solution was cooled to 4°C overnight. After aging, 1 mL of β-CD-AuNP stock solution was mixed with 200 µl of sodium carbonate-sodium bicarbonate buffer (0.2 M, pH 9.2) and 75 µl of MUA solution (0.1 M in ethanol) for 2 h to obtain Au NC1 was purified by centrifugation with a 10KDa cut-off ultrafiltration tube for 20min. Store at 4°C until use.
[0025] Synthesis of Au NC2 (β-CD and His bifunctional nanoclusters): HAuCl at room temperature 4 The aqueous solution (1 mL, 10 mM) was mixed with histidine (3 mL, 0.1 M) in a via...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com