Method for measuring N-substitution degree, total substitution degree, and acetylation degree of hydroxypropyl chitosan
A technology of hydroxypropyl chitosan and hydroxypropyl, which is applied in the research and application field of hydroxypropyl chitosan, which can solve the problems of small integral measurement value, incapable of single-peak measurement, easy introduction of errors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
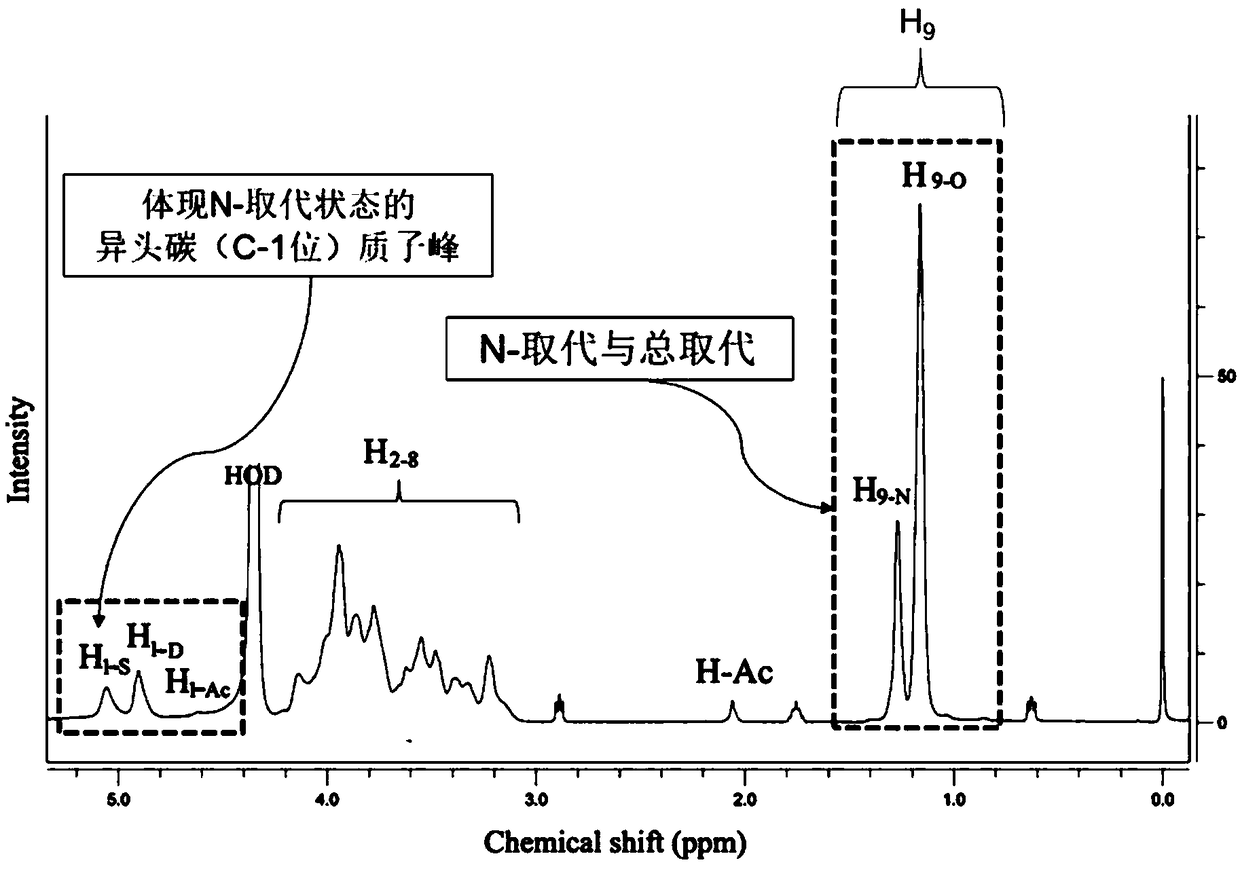
[0053] Reagents: The sample of hydroxypropyl chitosan is self-made in the laboratory, with shrimp chitosan (deacetylation degree 92.0%, made by 1 Measured by HNMR) as the raw material, prepared according to Peng's method "Preparation and antimicrobial activity of hydroxypropyl chitosan, Carbohydrate Research, 2005, 340:1846–1851".
[0054] Instrument: DD2500Mhz Nuclear Magnetic Resonance Instrument Agilent Technologies Co., Ltd.
[0055] experimental method:
[0056] A method for determining the degree of substitution of hydroxypropyl chitosan includes the following steps:
[0057] (1) Preparation of hydroxypropyl chitosan sample
[0058] a. Dry the sample of hydroxypropyl chitosan to remove moisture, the specific operation is: weigh 8mg sample of hydroxypropyl chitosan in a centrifuge tube, dry at 80°C for 4-5 hours, and then heat to 105°C until dry To constant weight. After drying, open the oven and seal it immediately, and place it in a desiccator to cool for later use;
[0059] b. ...
Embodiment 2
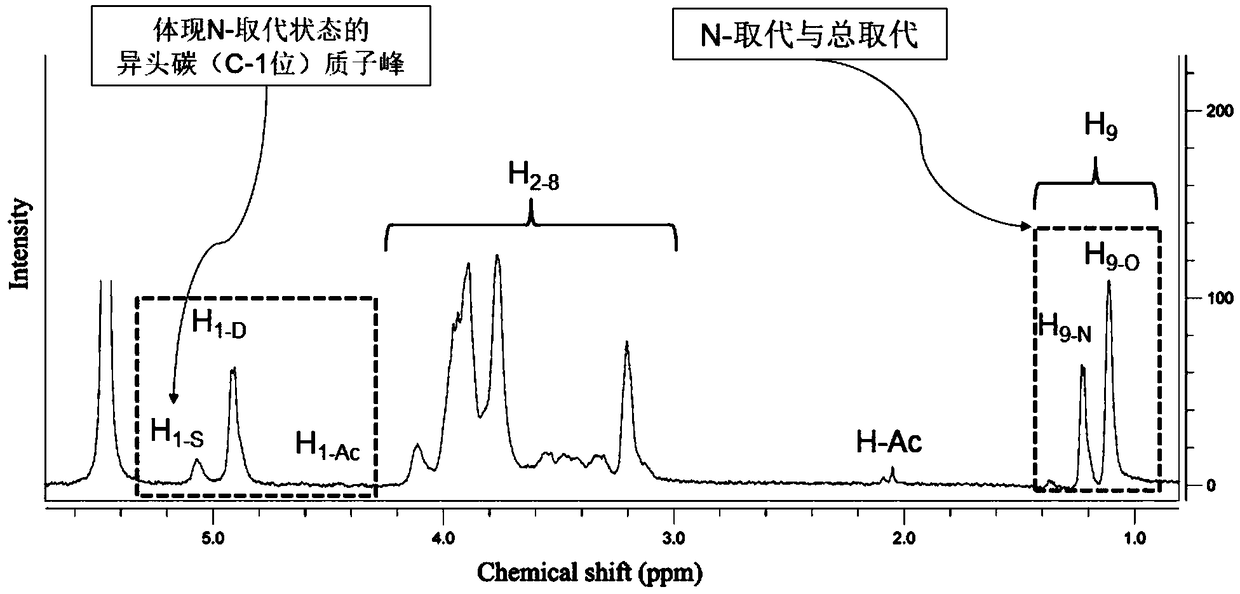
[0087] Reagents: The sample of hydroxypropyl chitosan is self-made in the laboratory, with shrimp chitosan (deacetylation degree 94.9% 1 Measured by HNMR) as the raw material, prepared according to Peng's method "Preparation and antimicrobial activity of hydroxypropyl chitosan, Carbohydrate Research, 2005, 340:1846–1851".
[0088] Instrument: Pro pulse 500MHz Agilent
[0089] experimental method:
[0090] A method for determining the degree of substitution of hydroxypropyl chitosan includes the following steps:
[0091] (1) Preparation of hydroxypropyl chitosan sample
[0092] a. Dry the sample of hydroxypropyl chitosan to remove water, the specific operation method is: weigh 8mg sample of hydroxypropyl chitosan in a centrifuge tube, dry at 80°C for 4-5 hours, and then heat to 105°C until Dry to constant weight. After drying, open the oven and seal it immediately, and place it in a desiccator to cool for later use;
[0093] b. Add sample
[0094] The deuterated reagent (from 500 μl D 2 ...
Embodiment 3
[0121] Reagents:
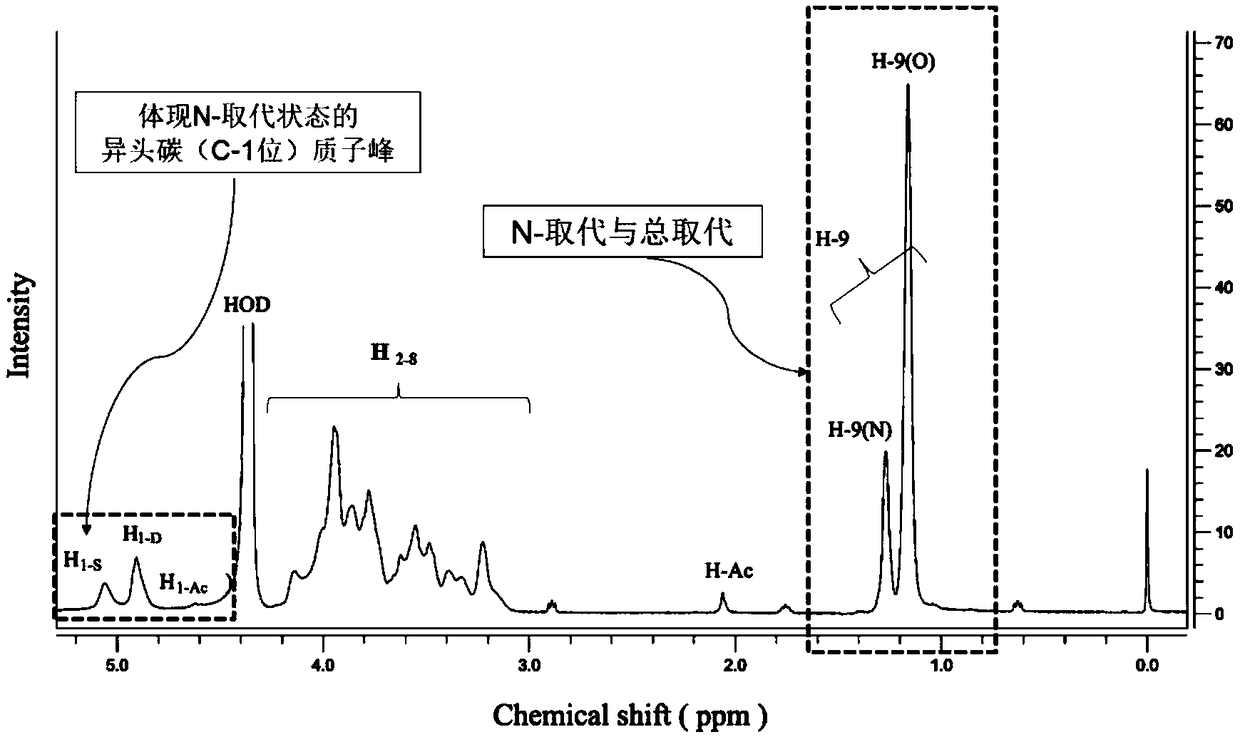
[0122] Reagents: The sample of hydroxypropyl chitosan was made in the laboratory, with shrimp chitosan (deacetylation degree of 92.9%, made by 1 Measured by HNMR) as the raw material, prepared according to Peng's method "Preparation and antimicrobial activity of hydroxypropyl chitosan, Carbohydrate Research, 2005, 340:1846–1851".
[0123] Instrument: JNM-ECP 600MHz Nuclear Magnetic Resonance System, JEOL Ltd.
[0124] experimental method:
[0125] A method for determining the degree of substitution of hydroxypropyl chitosan includes the following steps:
[0126] (1) Preparation of hydroxypropyl chitosan sample
[0127] a. Dry the sample of hydroxypropyl chitosan to remove water. The specific operation method is: weigh 7.5 mg of sample of hydroxypropyl chitosan in a centrifuge tube, dry it at 80°C for 4-5 hours, and then heat it to 105°C. Until dry to constant weight. After drying, open the oven and seal it immediately, and place it in a desiccator to cool for later u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com