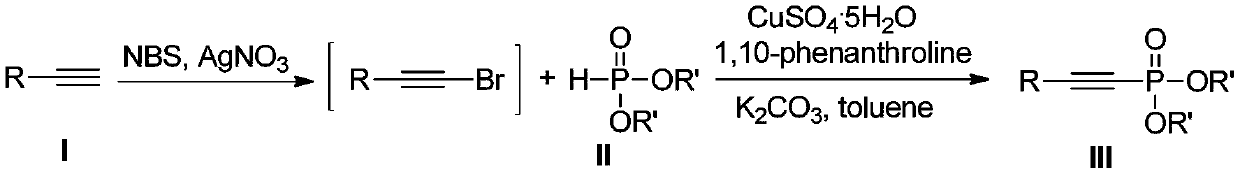
A kind of preparation method of alkynyl phosphate
A technology of alkynyl phosphate and terminal alkyne is applied in the field of preparation of alkynyl phosphate to achieve the effects of high reaction efficiency and simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
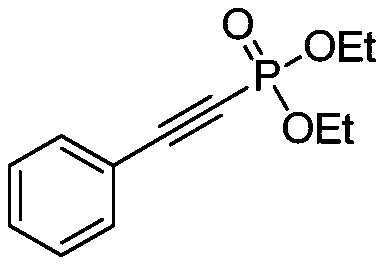
[0021] Example 1: Preparation of phenylethynyl diethyl phosphate
[0022] Under the protection of nitrogen, dissolve phenylacetylene (1mmol, 102mg) in acetone (1mL), then add silver nitrate (0.1mmol, 16.8mg) and NBS (1.2mmol, 211mg), stir the reaction mixture, react for 1h, filter and remove Remove the insoluble matter, spin dry, add diethyl phosphite (1mmol, 138mg), copper sulfate pentahydrate (0.1mmol, 24.8mg), 1,10-phenanthroline (0.2mmol, 36.0mg), potassium carbonate (2mmol 276mg), toluene (2mL), reacted at 65°C for 12 hours, filtered, the filter residue was washed with dichloromethane, spin-dried, and separated by column chromatography to obtain 204.7mg of a colorless liquid product with a yield of 86%.
[0023]
[0024] 1 H NMR(500MHz, CDCl 3 ): δ7.56(d,J=10.0Hz,2H),7.46-7.40(m,1H),7.39-7.36(m,2H),4.25-4.21(m,4H),1.41(t,J=5.0 Hz, 6H).
Embodiment 2
[0025] Example 2: Preparation of phenylethynyl dimethyl phosphate
[0026] Under the protection of nitrogen, dissolve phenylacetylene (1mmol, 102mg) in acetone (1mL), then add silver nitrate (0.1mmol, 16.8mg) and NBS (1.2mmol, 211mg), stir the reaction mixture, react for 1h, filter and remove Remove the insoluble matter, spin dry, add dimethyl phosphite (1mmol, 110mg), copper sulfate pentahydrate (0.1mmol, 24.8mg), 1,10-phenanthroline (0.2mmol, 36.0mg), potassium carbonate (2mmol 276mg), toluene (2mL), reacted at 65°C for 12 hours, filtered, the filter residue was washed with dichloromethane, spin-dried, column chromatography to obtain a colorless liquid product 174.3mg, the yield was 83%.
[0027]
[0028] 1 H NMR(500MHz, CDCl 3 ): δ7.58(d,J=10.0Hz,2H), 7.48-7.45(m,1H), 7.40-7.37(m,2H), 3.88(s,3H), 3.85(s,3H).
Embodiment 3
[0029] Example 3: Preparation of di-n-butyl phenylethynyl phosphate
[0030] Under the protection of nitrogen, dissolve phenylacetylene (1mmol, 102mg) in acetone (1mL), then add silver nitrate (0.1mmol, 16.8mg) and NBS (1.2mmol, 211mg), stir the reaction mixture, react for 1h, filter and remove Remove the insoluble matter, spin dry, add di-n-butyl phosphite (1mmol, 194mg), copper sulfate pentahydrate (0.1mmol, 24.8mg), 1,10-phenanthroline (0.2mmol, 36.0mg), potassium carbonate ( 2mmol, 276mg), toluene (2mL), reacted at 65°C for 12 hours, filtered, the filter residue was washed with dichloromethane, spin-dried, and separated by column chromatography to obtain a colorless liquid product 220.5mg with a yield of 75%.
[0031]
[0032] 1 H NMR(400MHz, CDCl 3 ): δ7.54(d,J=8.0Hz,2H),7.46-7.40(m,1H),7.39-7.35(m,2H),4.16-4.08(m,4H),1.75-1.68(m,4H) ),1.48-1.40(m,4H),0.94(t,J=8.0Hz,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com