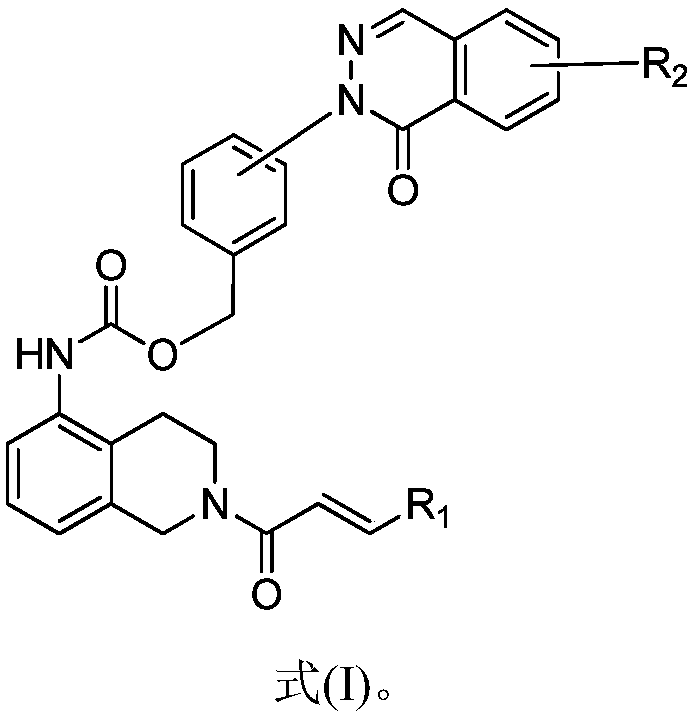
Pyridazinone BTK inhibitors and application thereof
A solvate and compound technology, applied in the field of medicinal chemistry, can solve problems such as unsatisfactory selectivity, drug resistance, and multiple side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 (3,4-dihydroisoquinoline-2(1H)-formic acid tert-butyl ester-5-yl)-carbamic acid o-chlorobenzyl ester
[0034]
[0035] Weigh 5-amino-3,4-dihydroisoquinoline-2(1H)-tert-butyl carboxylate (50mmol) and DIPEA (100mmol) into a reaction flask, add 300ml of dichloromethane, and slowly add it dropwise under stirring at room temperature O-chlorobenzyl chloroformate (51mmol), after dropping, continue to stir at room temperature for 1h, stop the reaction, concentrate the reaction mixture, add 70ml of ethyl acetate, wash with dilute aqueous hydrochloric acid (0.2-0.3N) and saturated brine, anhydrous sulfuric acid Dry over sodium, filter, and concentrate to give the title compound, which is used directly in the next step.
[0036] ESI–MS:[M+H] + m / z 417.
Embodiment 2
[0037] Example 2 Preparation of (3,4-dihydroisoquinoline-2(1H)-formic acid tert-butyl ester-5-yl)-carbamic acid p-chlorobenzyl ester
[0038]
[0039] Weigh triphosgene (5mmol) into a reaction bottle, add 100ml of toluene, add dropwise 20ml of tetrahydrofuran solution dissolved with p-chlorophenol (5mmol) and pyridine (10ml) at 0°C, after the drop is completed, continue to react at room temperature for 8h, concentrate the reaction solution, added 40ml of dichloromethane, suspended to dryness, and obtained p-chlorobenzyl chloroformate, which was directly used in the next step.
[0040] Using tert-butyl 5-amino-3,4-dihydroisoquinoline-2(1H)-carboxylate and p-chlorobenzyl chloroformate as raw materials, the title compound was obtained by the same method as in Example 1.
[0041] ESI–MS:[M+H] + m / z 417.
Embodiment 3
[0042] Example 3 Preparation of 2H-phthalazin-1-one
[0043]
[0044] Step 1: Weigh dimethoxymethylbenzene (500mmol) into a reaction flask, add tetrahydrofuran (800ml) to dissolve, add s-BuLi (565mmol) under nitrogen protection at 60°C, and store the reaction solution at -60°C Stir for 1h.
[0045] Step 2: Weigh dry ice (50mmol) into a reaction flask, add tetrahydrofuran (200ml), add n-BuLi (5ml), stir for 2h under nitrogen protection, add the mixture of step 1, continue stirring for 30min, stop the reaction, add water 1000ml, adjust the pH to 2 with concentrated hydrochloric acid, separate the organic phase, extract the aqueous phase with ethyl acetate, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, and recrystallize to obtain 2-dimethoxymethylbenzoic acid .
[0046] Step 3: Weigh the product obtained in Step 2 (400mmol), acetic acid (93mmol), and hydrazine (600mmol) into a reaction flask, add 300ml of isopropanol, under nitrogen ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com