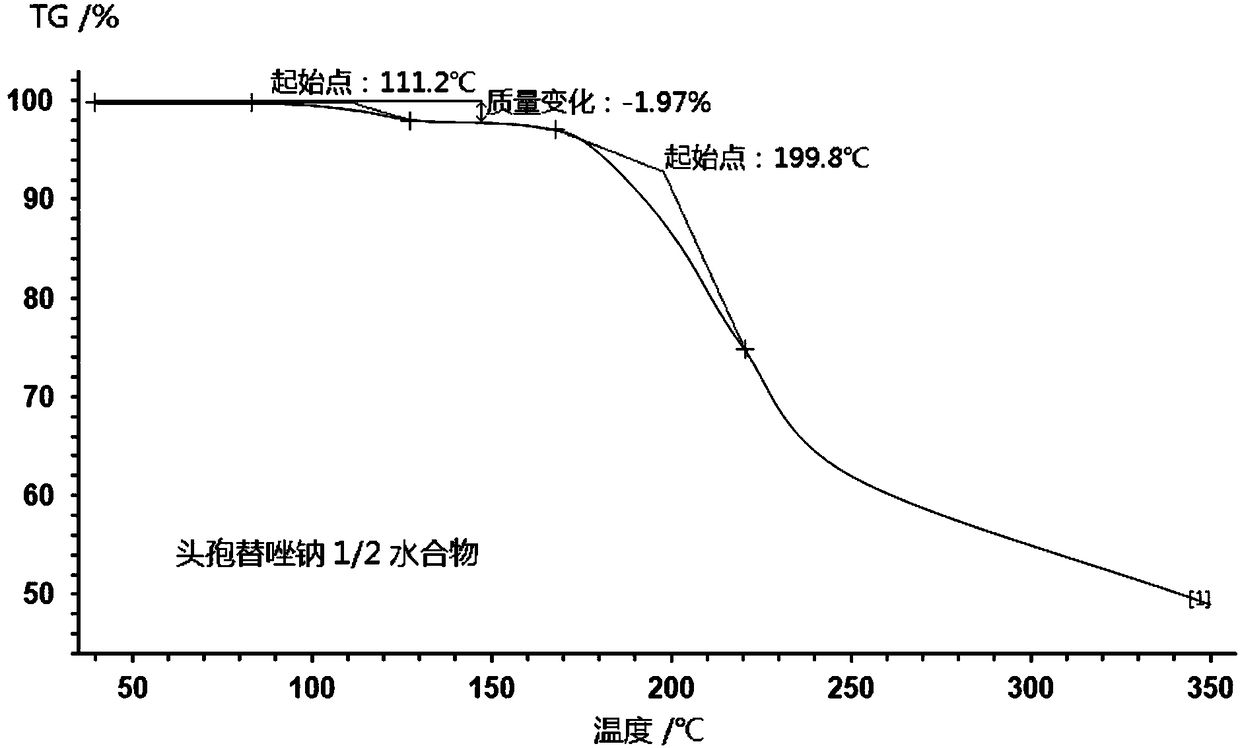
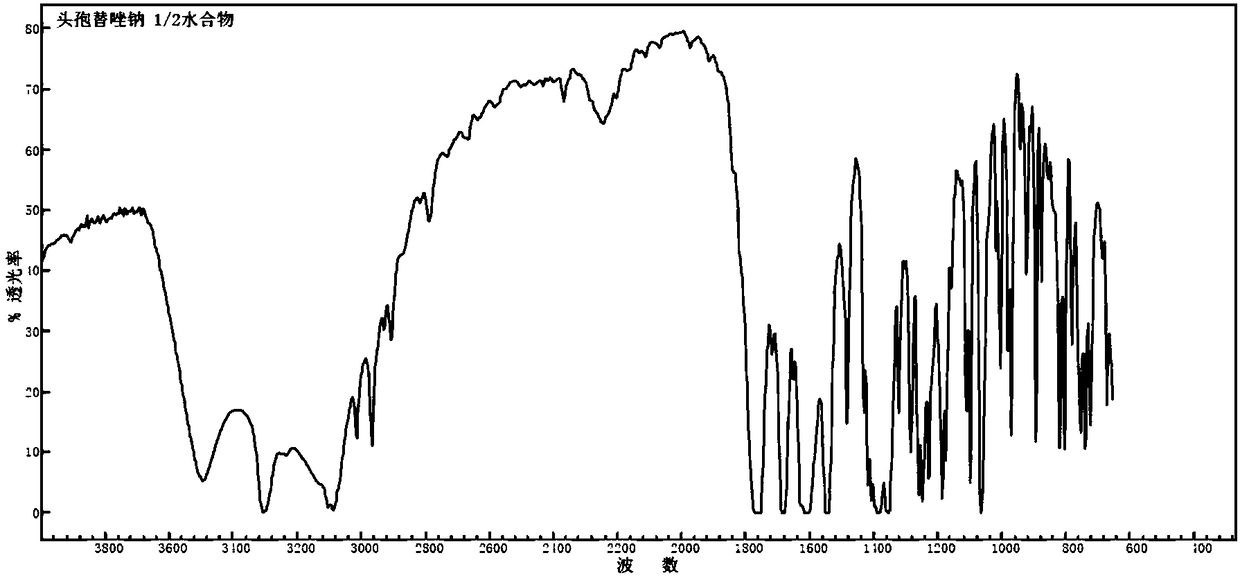
1/2 water ceftezole sodium compound
A technology of ceftazole sodium and compound, applied in the field of 1/2 water ceftazole sodium compound and preparation method thereof, can solve the problems of poor fluidity and high hygroscopicity of ceftazole sodium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of 1 / 2 water ceftezole sodium compound
[0029] Measure 600ml of isopropanol and 100ml of water into the reaction kettle, under stirring, add 60.03g of ceftezole, control the temperature at 25°C, then slowly add sodium acetate, when the pH value is 7, stop adding, add 0.61g of activated carbon , decolorize by adsorption for 30min, decarbonize by filtration, wash the filter cake with 50ml of isopropanol×2; add the filtrate to the reaction kettle, add 500ml of ether under slow stirring, lower the temperature to 5°C, add dropwise 100ml of 2:1 ethanol and butyl The ketone mixture was stirred and crystallized for 2 hours, filtered with suction, the filter cake was washed with 50ml of ethanol × 2, and dried under reduced pressure at 40°C for 1.5 hours to obtain 46.9g of 1 / 2 water ceftezole sodium compound.
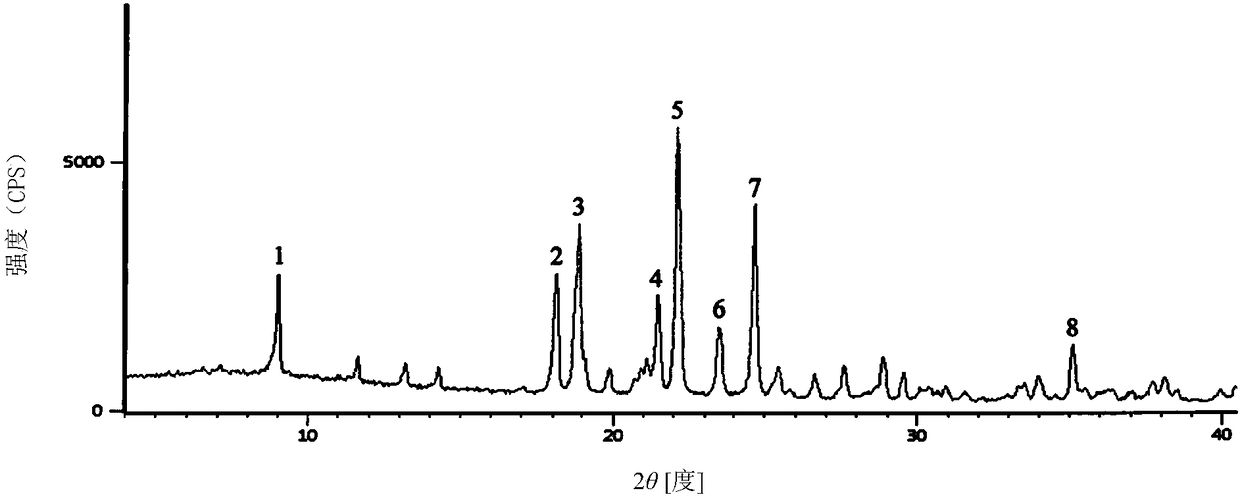
[0030] The powder X-ray diffraction pattern has characteristic diffraction peaks at the diffraction angles 2θ of 9.02°, 18.15°, 18.89°, 21.5...
Embodiment 2
[0033] Embodiment 2: Preparation of 1 / 2 water ceftezole sodium compound
[0034] Measure 700ml of isopropanol and 100ml of water into the reaction kettle, under stirring, add 60.11g of ceftezole, control the temperature at 15°C, then slowly add sodium acetate, when the pH value is 8, stop adding, add 0.63g of activated carbon , adsorption and decolorization for 30min, decarbonization by filtration, and washing the filter cake with 50ml of isopropanol×2; put the filtrate into the reaction kettle, add 500ml of diethyl ether under slow stirring, lower the temperature to 10°C, and dropwise add 100ml of 3:1 ethanol and butyl The ketone mixture was stirred and crystallized for 2.5 hours, filtered with suction, the filter cake was washed with 50ml of ethanol × 2, and dried under reduced pressure at 45°C for 2 hours to obtain 45.5g of 1 / 2 water ceftezole sodium compound.
[0035] The powder X-ray diffraction pattern has characteristic diffraction peaks at the diffraction angles 2θ of ...
Embodiment 3
[0038] Embodiment 3: Preparation of 1 / 2 water ceftezole sodium compound
[0039] Measure 800ml of isopropanol and 100ml of water into the reaction kettle, under stirring, add 60.07g of ceftezole, control the temperature at 35°C, then slowly add sodium acetate, stop adding when the pH value is 7.5, and add 0.64g of activated carbon , adsorption and decolorization for 30 minutes, decarbonization by filtration, washing the filter cake with 50ml of isopropanol×2; adding the filtrate to the reaction kettle, adding 500ml of ether under slow stirring, lowering the temperature to 15°C, and adding dropwise 100ml of 2.5:1 ethanol and butyl The ketone mixture was stirred and crystallized for 3 hours, filtered with suction, the filter cake was washed with 50ml of ethanol × 2, and dried under reduced pressure at 50°C for 1 hour to obtain 47.3g of 1 / 2 water ceftezole sodium compound.
[0040] The powder X-ray diffraction pattern has characteristic diffraction peaks at the diffraction angles...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com