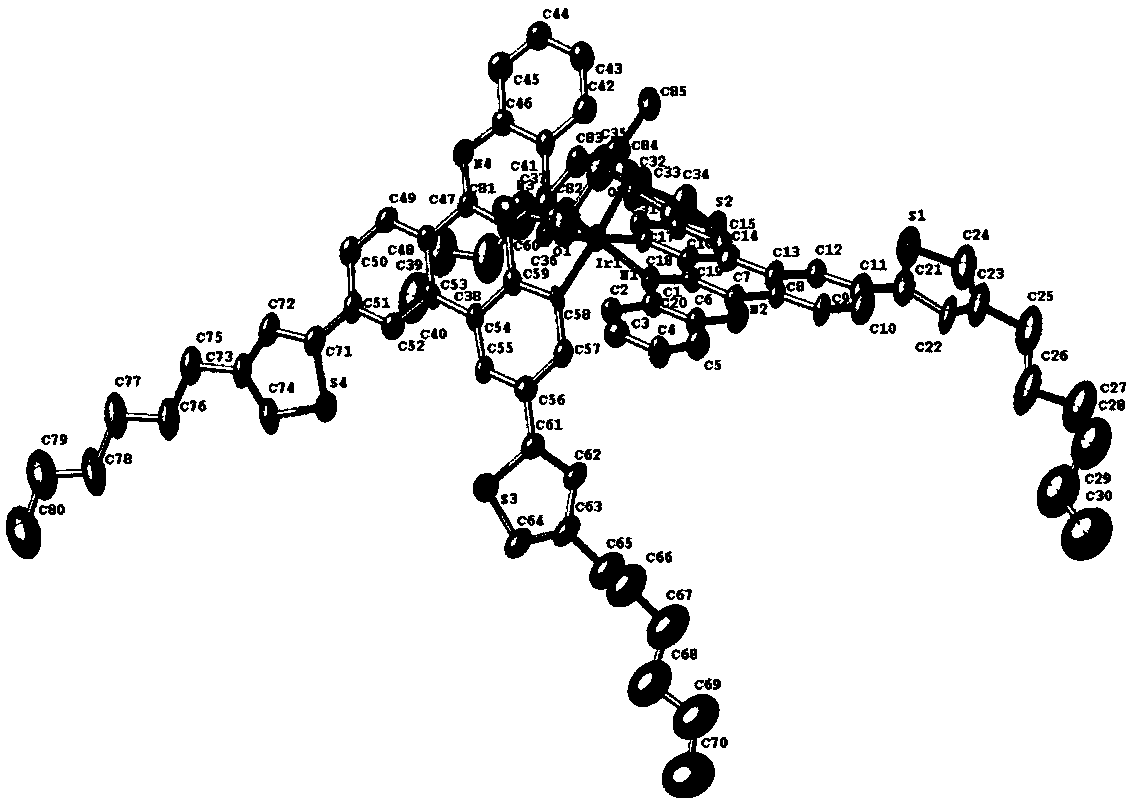
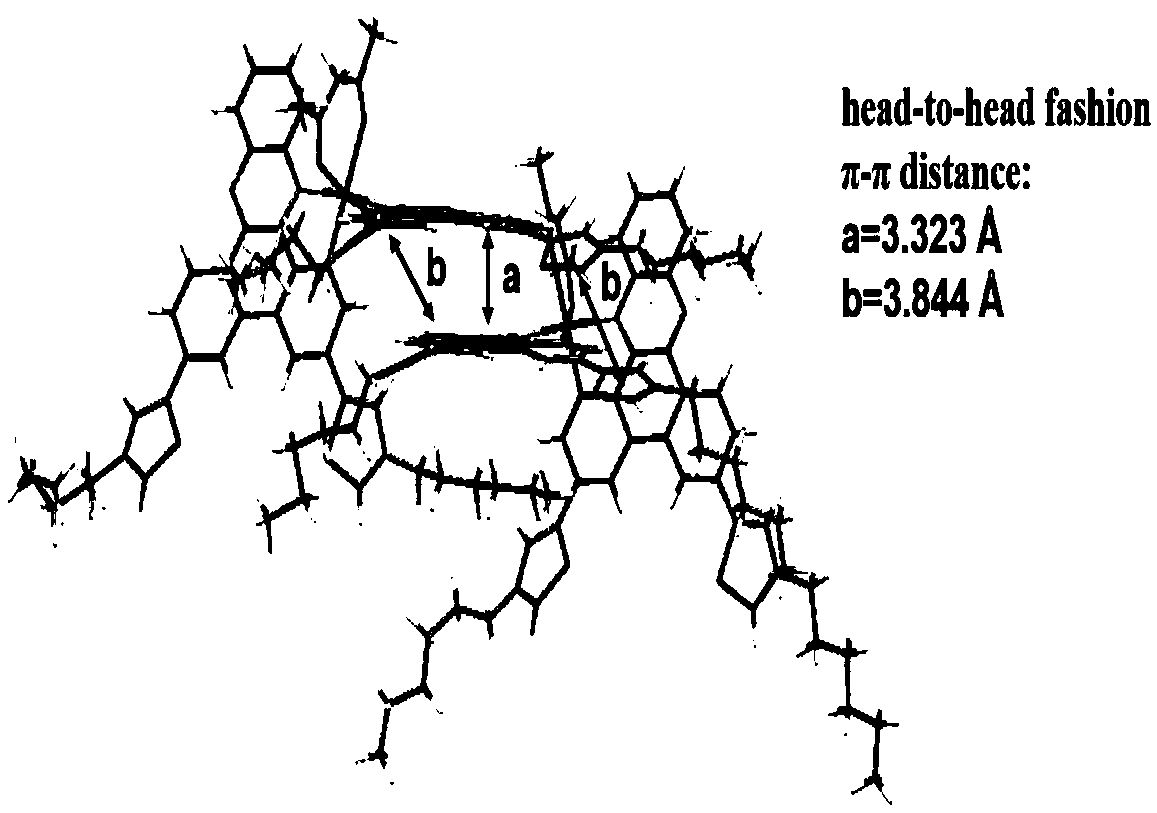
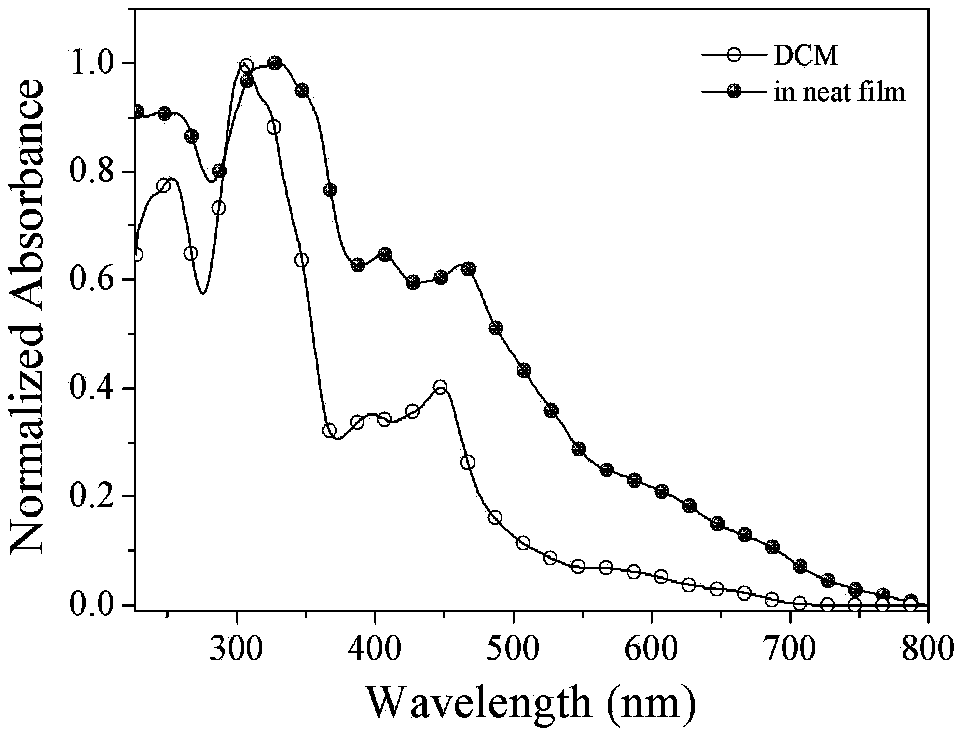
D-A-D cyclometalated iridium complex near-infrared luminous material based on aryl quinoxaline and application
A technology of D-A-D and iridium complexes, which can be applied to luminescent materials, compounds containing elements of group 8/9/10/18 of the periodic table, indium organic compounds, etc., and can solve problems such as low luminous efficiency and unclear structure-activity relationship , to achieve the effects of simple synthesis steps, easy purification, good solubility and film-forming properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of D-A-D Cyclometallic Iridium Complex Ir-1 Based on Arylquinoxaline
[0049] The synthetic route of iridium complex Ir-1 is as follows:
[0050]
[0051] Synthesis of Intermediate 1
[0052] In a 100mL single-necked bottle, add 1,2-phenylenediamine (325mg, 3.00mmol), 3,6-dibromo-9,10-phenanthrenequinone (1g, 2.73mmol) and absolute ethanol (30mL), acetic acid (1mL), heated at 80°C, stirred at reflux for 4h, and a large amount of yellow-green solid precipitated. Cool to room temperature, filter with suction, wash the filter cake with absolute ethanol (10×3 mL) and vacuum-dry for 3 hours to obtain 1.19 g of a yellow-green solid with a yield of 91%, which is directly put into the next reaction.
[0053] Synthesis of Intermediate 2
[0054] In a 100mL two-necked flask, sequentially add intermediate 1 (500g, 1.14mmol), (4-hexylthienyl)tributyltin (1.56g, 3.42mmol), tetrakis(triphenylphosphine)palladium (40mg, 0.034mmol ), toluene (20mL), heated to 80°C und...
Embodiment 2
[0059] Preparation of D-A-D Cyclometallic Iridium Complex Ir-2 Based on Arylquinoxaline
[0060] The synthetic route of iridium complex Ir-2 is as follows:
[0061]
[0062] Synthesis of Intermediate 3
[0063] In a 100mL two-necked flask, intermediate 1 (500g, 1.14mmol), triphenylamine 4-boronate (824mg, 2.85mmol), tetrakis(triphenylphosphine) palladium (40mg, 0.034mmol) were added in turn in Example 1 ), 2mol / L potassium carbonate solution (5mL), absolute ethanol (1mL), toluene (25mL), under nitrogen atmosphere, heated to 80°C, and stirred for 12h. The toluene was distilled off under reduced pressure, and the remaining liquid was extracted with dichloromethane, washed with water, anhydrous MgSO 4 After drying and filtering, the filtrate was distilled off under reduced pressure to remove the solvent, and the residue was separated and purified through a silica gel column using a mixed solution of dichloromethane and petroleum ether (1 / 2, V / V) as an eluent to obtain 716 mg...
Embodiment 3
[0067] Preparation of D-A-D Cyclometallic Iridium Complex Ir-3 Based on Arylquinoxaline
[0068] The synthetic route of iridium complex Ir-3 is as follows:
[0069]
[0070] Synthesis of Intermediate 4
[0071] In a 100mL two-necked flask, add 4-triphenylamine borate (1.40g, 4.70mmol), 4,5-dibromo-o-phenylenediamine (0.50g, 1.88mmol), tetrakis(triphenylphosphine) palladium ( 130mg, 0.029mmol), 2mol / L potassium carbonate solution (8mL), toluene (20mL), under nitrogen atmosphere, heated to 80°C, and stirred for 12h. The toluene was distilled off under reduced pressure, and the remaining liquid was extracted with dichloromethane, washed with water, anhydrous MgSO 4 After drying and filtering, the filtrate was distilled off under reduced pressure to remove the solvent, and dried in vacuo for 3 hours to obtain 894 mg of white solid with a yield of 80%. Because the product is extremely sensitive to oxygen, it was directly put into the next reaction without purification.
[0072...
PUM
| Property | Measurement | Unit |
|---|---|---|
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com