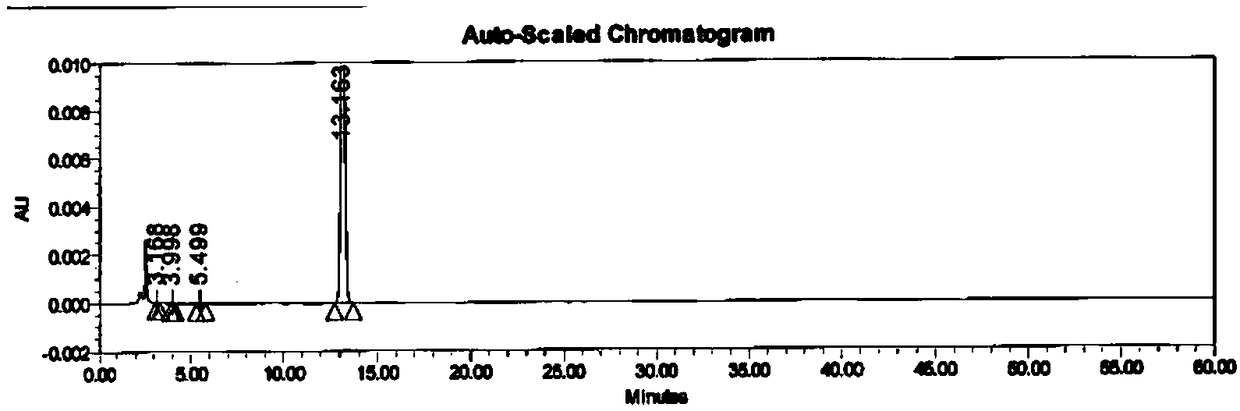
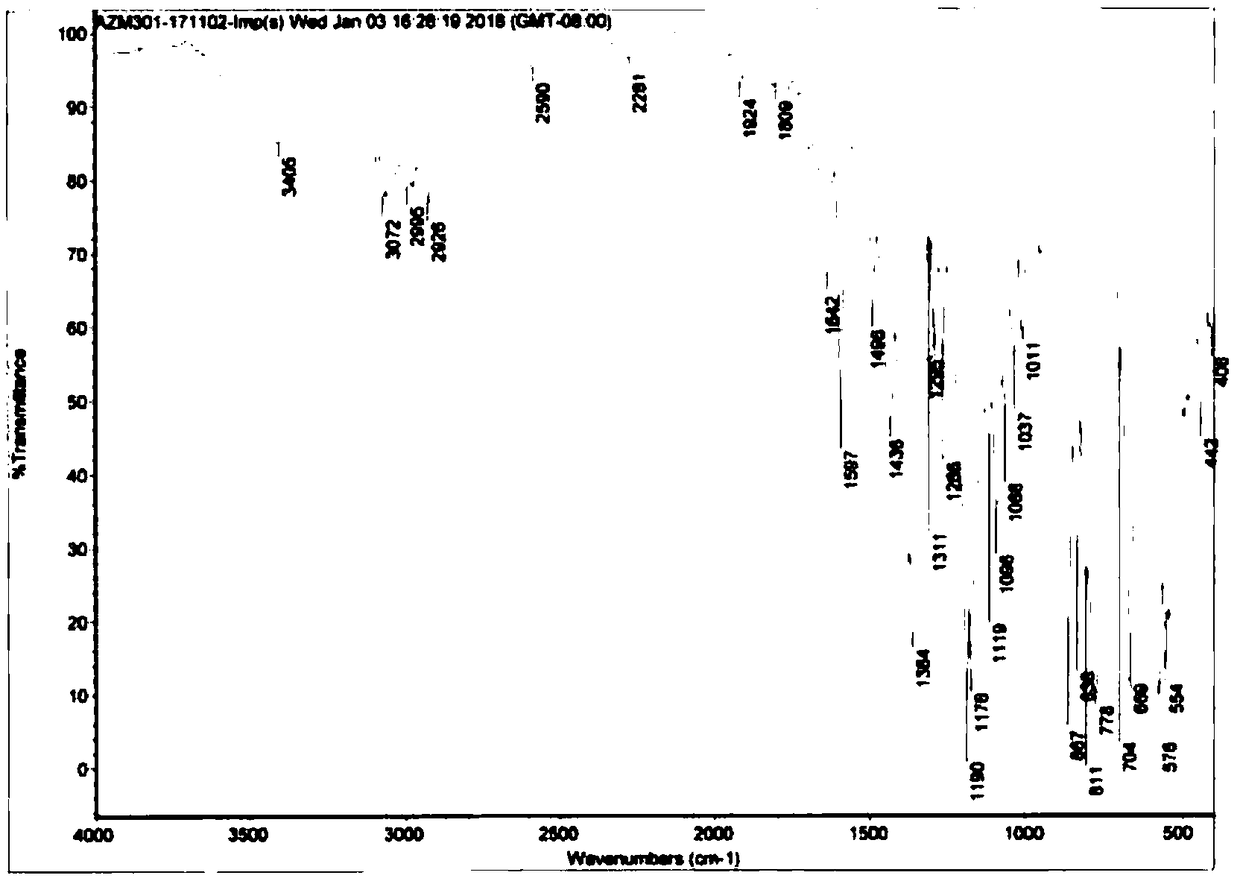
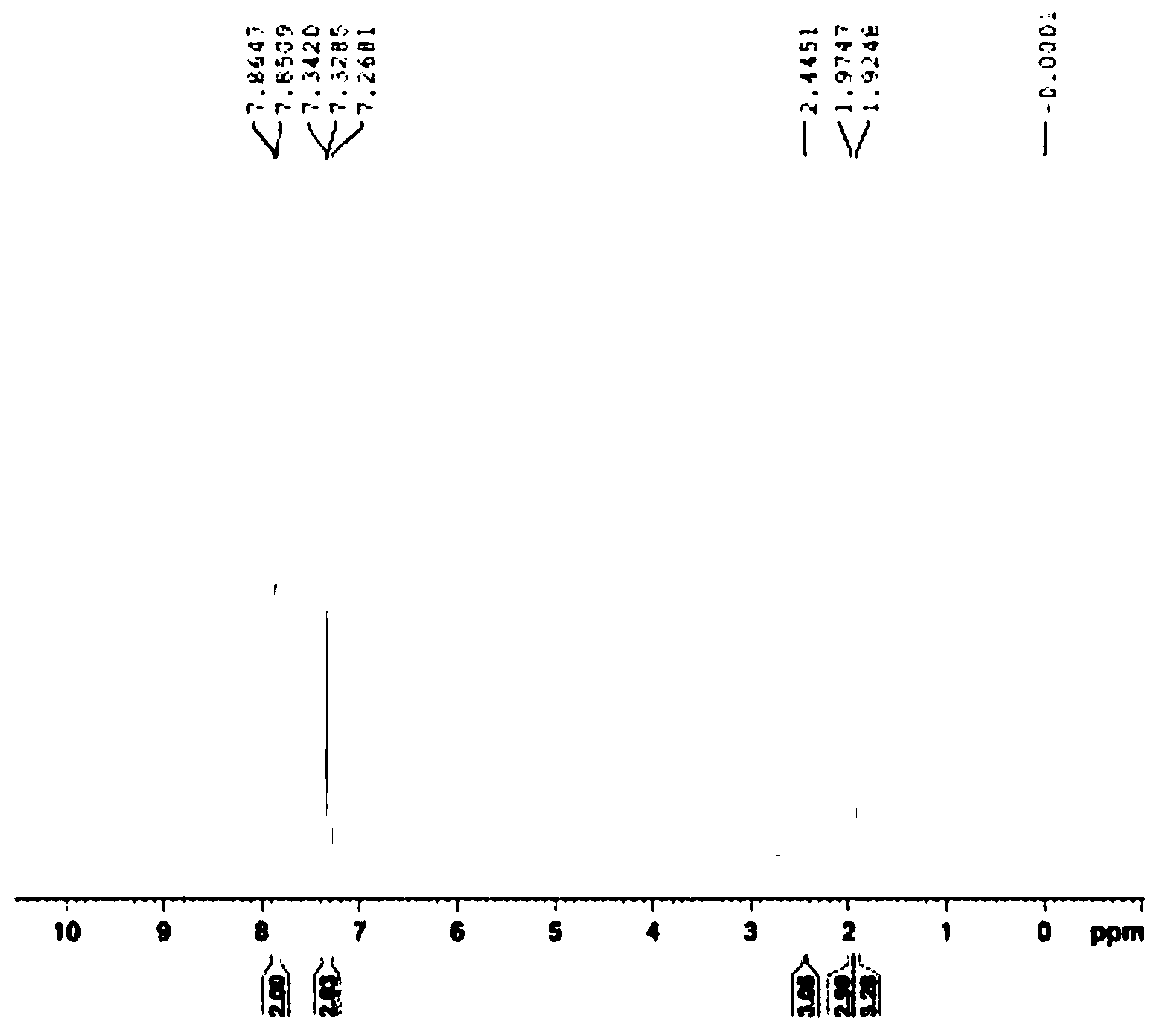
Synthetic method of azithromycin genotoxic impurities
A technology of genotoxicity and synthesis method, applied in the field of chemistry, to achieve the effect of improving quality, high yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The synthesis of embodiment 1 acetone oxime-O-p-methylsulfonate
[0033] Take 20g of hydroxylamine hydrochloride (compound II, 0.29mol), 200ml (2.7mol) of acetone, control the temperature at 10°C, add 80g (0.95mol) of sodium bicarbonate, stir and react for 0.8h, then add 20g (0.10mol) of p-toluenesulfonyl chloride mol), continue to control the temperature at 10°C, react for 4h, add 400ml of water and stir to precipitate a large amount of solid, filter with suction, and dry under reduced pressure at 35°C for 4h to obtain 18.4g (0.08mol) of white solid, yield 92.0%.
[0034] Add 10 g of the crude product of acetone oxime-O-p-methylsulfonate into a single-necked bottle, add 100 ml of 60% acetone aqueous solution, stir at 0-10 ° C for 1 h, filter with suction, and dry to obtain 9.1 g of white solid, with a yield of 91.0%. 99.7% purity.
Embodiment 2
[0035] The synthesis of embodiment 2 acetone oxime-O-p-methylsulfonate
[0036] Take 20g of hydroxylamine hydrochloride (compound II, 0.29mol), 200ml (2.7mol) of acetone, control the temperature at 0°C, add 40g (0.48mol) of sodium bicarbonate, stir and react for 1.5h, then add 15g (0.078mol) of p-toluenesulfonyl chloride mol), continue to control the temperature at 0°C, react for 2h, add 400ml of water and stir to precipitate a large amount of solid, filter with suction, and dry under reduced pressure at 35°C for 4 to obtain 13.7g (0.06mol) of white solid, yield 91.5%.
[0037] Add 10 g of the crude product of acetone oxime-O-p-methylsulfonate into a single-necked bottle, add 80 ml of 60% acetone aqueous solution, stir at 0-10 °C for 1 h, filter with suction, and dry to obtain 9.3 g of white solid, with a yield of 93.0%. 99.5% purity.
Embodiment 3
[0038] Embodiment 3, the synthesis of acetone oxime-O-p-methylsulfonate
[0039] Take 20g of hydroxylamine hydrochloride (compound II, 0.29mol), 200ml (2.7mol) of acetone, control the temperature at 5°C, add 60g (0.71mol) of sodium bicarbonate, stir and react for 1 hour, then add 20g (0.10mol) of p-toluenesulfonyl chloride ), continue to control the temperature at 5°C, react for 3h, add 400ml of water and stir to precipitate a large amount of solid, filter with suction, and dry under reduced pressure at 35°C for 4h to obtain 18.64g (0.08mol) of white solid with a yield of 93.2%.
[0040] Add 10 g of the crude product of acetone oxime-O-p-methylsulfonate into a single-necked bottle, add 100 ml of 60% acetone aqueous solution, stir for 1 h at 0-10 ° C, filter with suction, and dry to obtain 9.3 g of white solid, with a yield of 93.0%. 99.8% pure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com