Fluorescent microsphere-colloidal gold dual-color qualitative and quantitative immunochromatographic test strip for detecting clenbuterol hydrochloride and preparation method thereof
A technology of clenbuterol hydrochloride and immunochromatographic test paper, which is applied to measuring devices, analytical materials, instruments, etc., can solve the problems of large sample background interference, no longer accurate and reliable results, and long time-consuming, so as to eliminate false positive results , Realize the result data, avoid the effect of matrix interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Fluorescence microsphere-colloidal gold double color qualitative and quantitative immunochromatographic test strip preparation for detecting clenbuterol hydrochloride (optimum ratio of fluorescent microsphere and colloidal gold antibody labeling complex)
[0056] 1. Preparation process of immunochromatographic test strips
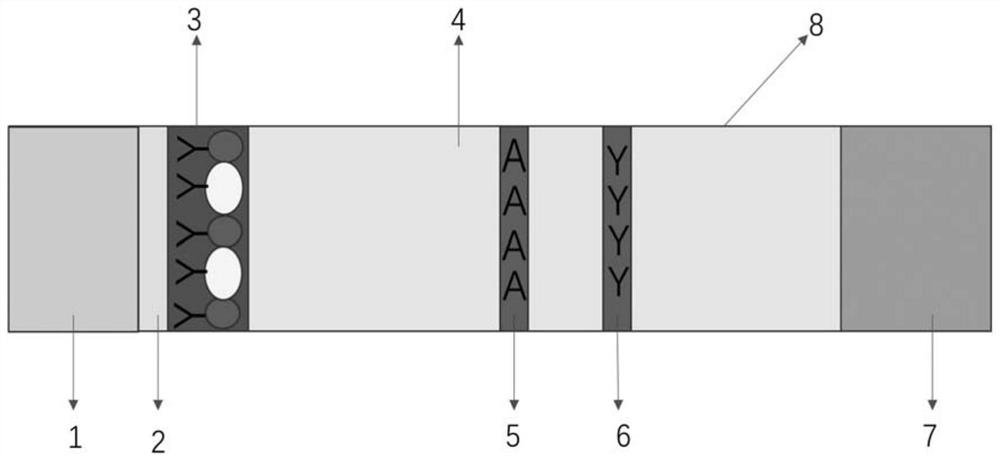
[0057] 1. Preparation of nitrocellulose membrane
[0058] (1) Preparation of clenbuterol hydrochloride artificial antigen (CLE-BSA)
[0059] The coupling method is the diazo method, the coupling protein is bovine serum albumin, and the coupling ratio is 1:10-1:100. After coupling, the CLE-BSA is obtained by dialysis and purification.
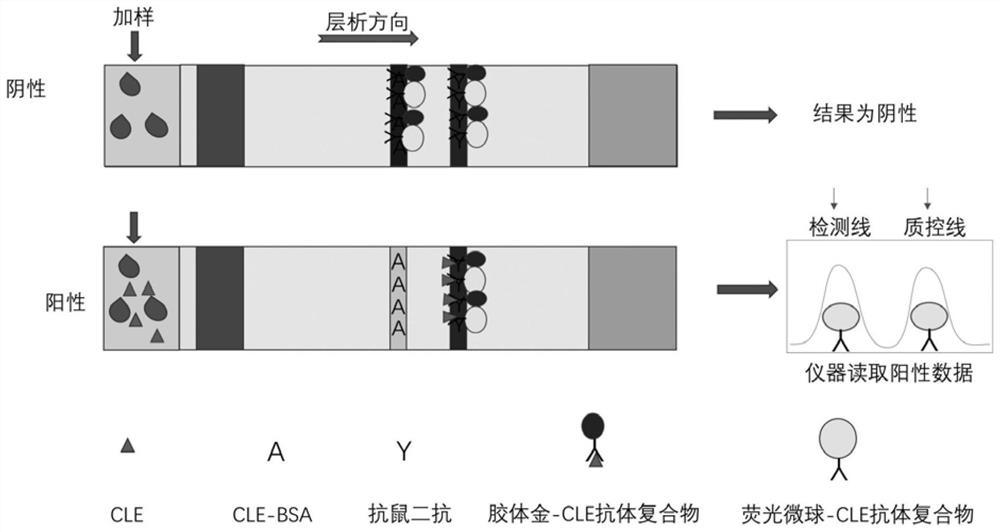
[0060] (2) Preparation of test line and quality control line
[0061] CLE-BSA conjugate and goat anti-mouse antibody are coated on nitrocellulose membrane: Dilute the CLE-BSA conjugate with 0.05M pH 7.2 PBS (phosphate buffered saline) to make the concentration 0.2mg / mL, The resulting solution was sprayed on the membra...
Embodiment 2
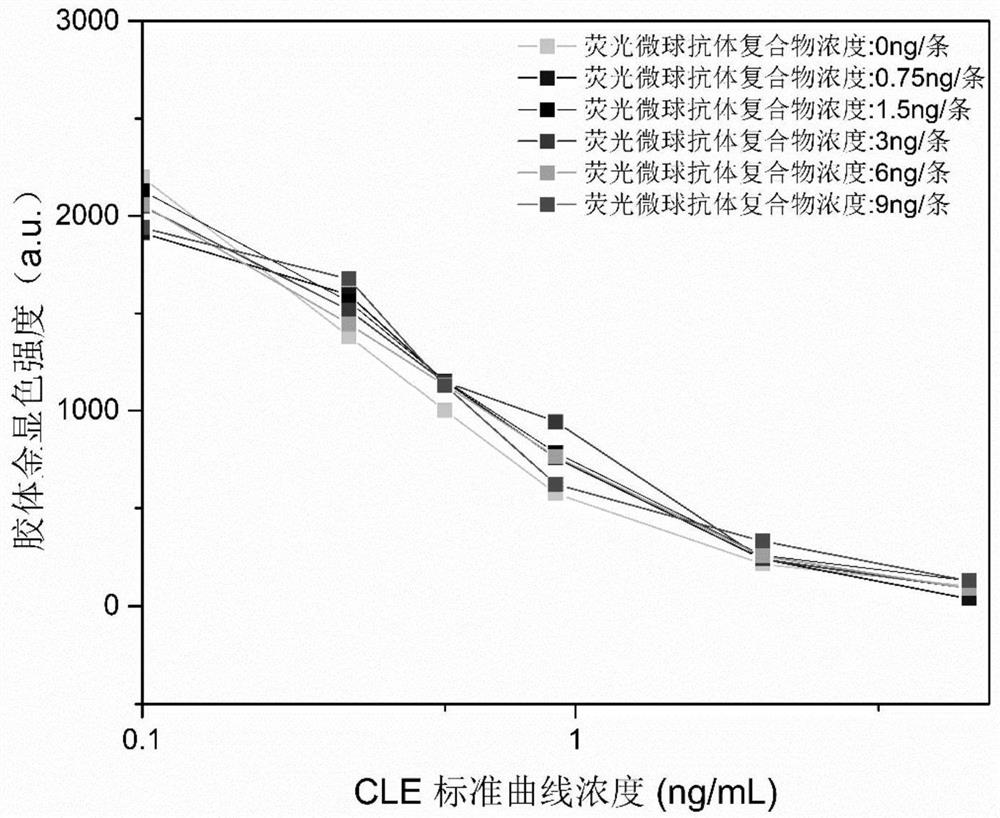
[0079] Example 2: Preparation of fluorescent microsphere-colloidal gold dual color qualitative and quantitative immunochromatographic test strip for detecting clenbuterol hydrochloride (the proportion of fluorescent microsphere labeling complexes increases)
[0080] The difference from Example 1 is:
[0081] (1) After the preparation of fluorescent microsphere antibody complex and colloidal gold antibody complex is completed, calculate according to the amount of antibody on different markers in each test strip, and the amount of colloidal gold antibody is accurate to 30ng / bar, fluorescent microsphere The antibody amount was 6 ng / strip marker concentration to spray the binding pad. Spray on 30×0.8cm glass fiber membrane, vacuum dry at 25°C for 1 to 2 hours, and place in a drying cabinet for later use. The quantitative detection results of fluorescent microspheres are: the concentration of the standard curve under this condition is: 0, 0.1, 0.3, 0.9, 1.5, 2.7 ng / mL, R 2 0.9058, IC 5...
Embodiment 3
[0085] Example 3: Preparation of fluorescent microsphere-colloidal gold double color qualitative and quantitative immunochromatographic test strip for detecting clenbuterol hydrochloride (the proportion of colloidal gold labeled complexes increases)
[0086] The difference from Example 1 is:
[0087] (1) After the preparation of the fluorescent microsphere antibody complex and the colloidal gold antibody complex is completed, calculate according to the amount of antibody on the different markers in each test strip, and the amount of colloidal gold antibody is accurate to 50ng / bar, fluorescent microspheres The amount of antibody was 3ng / strip marker concentration to spray the binding pad. Spray on 30×0.8cm glass fiber membrane, vacuum dry at 25°C for 1 to 2 hours, and place in a drying cabinet for later use. The quantitative detection result of fluorescent microspheres is: the concentration of the standard curve under this condition is: 0, 0.1, 0.3, 0.9, 1.5, 2.7 ng / mL, R 2 0.9694,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com