Amido-fullerene derivative and preparing method and application thereof
An aminofullerene and fullerene technology, applied in the field of aminofullerene derivatives, can solve the problems of low water solubility and easy agglomeration of fullerene derivatives, and achieve high water solubility and high scavenging freedom. The activity of the base, the effect of not easy to agglomerate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1: Preparation C 60 (EDA) 6
[0074] (a) Weigh 50 mg of solid fullerene C with an analytical balance 60 (Purity: 99%, Xiamen Funa New Material Technology Co., Ltd.) was dissolved in 25mL of o-xylene solution, ultrasonically dispersed for 30min, and 10mL of ethylenediamine (analytical grade, national medicine reagent) was measured with a measuring cylinder, and the o-xylene The solution and ethylenediamine were added to a 250mL Erlenmeyer flask with a stopper, then a magnet was added, and stirred for 1h using a magnetic stirrer (temperature: room temperature, speed: 1000r / min, N 2 as a protective gas), let it stand, and collect the precipitate.
[0075] (b) Add 50mL of ethylenediamine (analytical grade, Sinopharm Reagent) to the collected precipitate, and stir for 24 hours (temperature: room temperature, rotating speed: 1000r / min, N 2 as protective gas).
[0076] (c) Add the solution obtained in step (b) into a 250ml round bottom flask, and then use a rotary...
Embodiment 2
[0087] Example 2: Preparation C 70 (EDA) 8
[0088] Reaction conditions and steps are the same as in Example 1, except that 50mg of fullerene C is weighed 70 Solid (purity: 99%, Xiamen Funa New Material Technology Co., Ltd.) instead of C 60 . finally obtained with C 70 (EDA) 8 clear reddish-brown solution.
[0089] Using the same test method and conditions as in Example 1, the obtained product was tested for components.
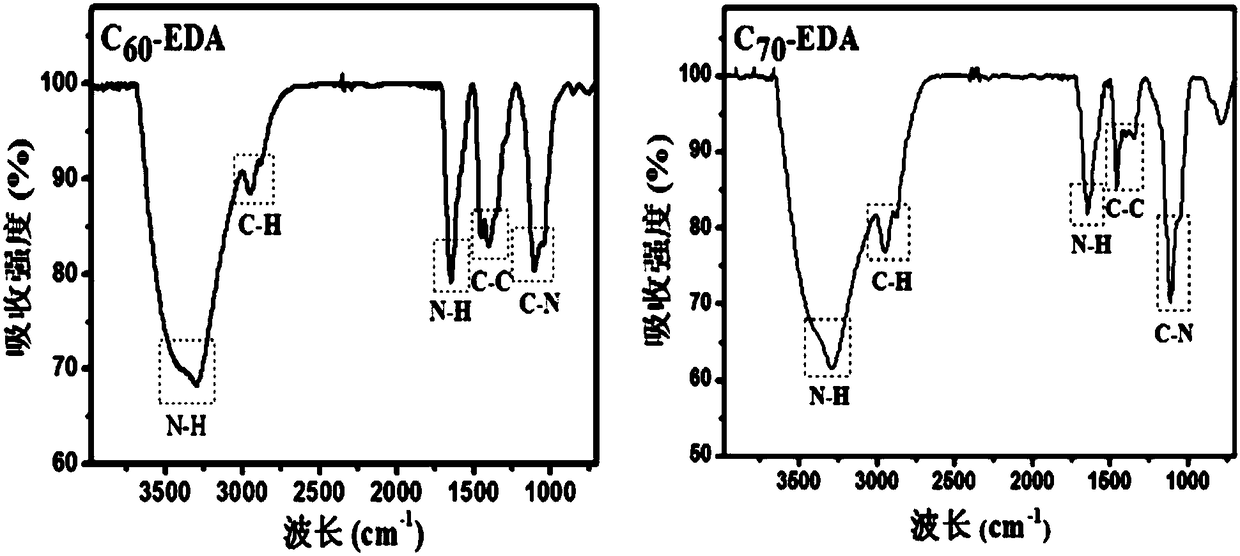
[0090] Such as figure 1 As shown, the characteristic infrared absorption of the sample around 3300nm (-NH 2 The stretching vibration absorption peak of the carbon cage) proves that ethylenediamine is bonded to the carbon cage of fullerene, and at the same time, the change of the infrared characteristic absorption at 800-1500nm (the stretching vibration peak of C-C and C=C on the carbon cage) also supports the change of ethylenediamine Already bonded to the carbon cage.
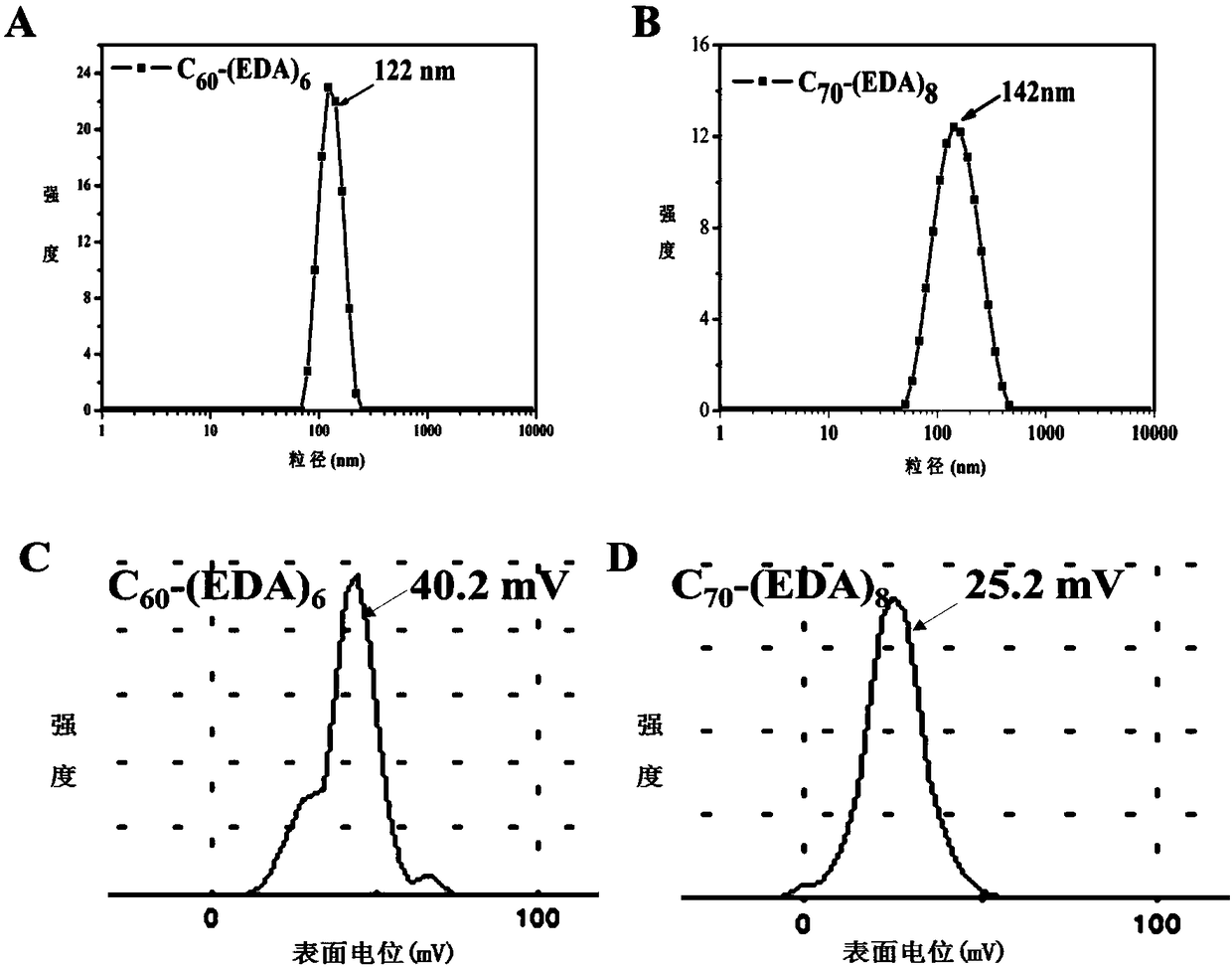
[0091] The sample solution is directly used in the dynamic light scattering (DL...
Embodiment 3
[0097] Example 3: C 60 (EDA) 6 、C 70 (EDA) 8 Hydroxyl radical scavenging test
[0098] Induction of H by ultraviolet (UV) 2 o 2 Hydroxyl radicals are generated, and DMPO is used as a free radical scavenger. DMPO quickly combines with free hydroxyl radicals to generate DMPO-OH, which can be detected by EPR.
[0099] Solution preparation: 100 μL of DMPO (100 mM) and 50 μL of HO 2 o 2 (100mM) and 50 μL of ultrapure water were mixed to prepare a blank control group.
[0100] test C 60 (EDA) 6、 C 70 (EDA) 8 When scavenging free radicals, the concentrations of samples added to the test system are: 20, 50, 100, 200, 400 μM.
[0101] see test results image 3 . C 60 (EDA) 6 、C 70 (EDA) 8 When the concentration is 50μM, there is an obvious effect of scavenging free radicals, when C 60 (EDA) 6 、C 70 (EDA) 8 When the concentration is 400 μM, the effect of scavenging hydroxyl free can reach 90%, and there is an obvious dose-effect relationship. For the convenience o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com