Application of amino fullerenes in preparing antibacterial materials and antibacterial material
A technology of aminofullerene and antibacterial material is applied to the application of aminofullerene in the preparation of antibacterial materials and the field of antibacterial materials, and can solve the problems of non-selective antibacterial and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
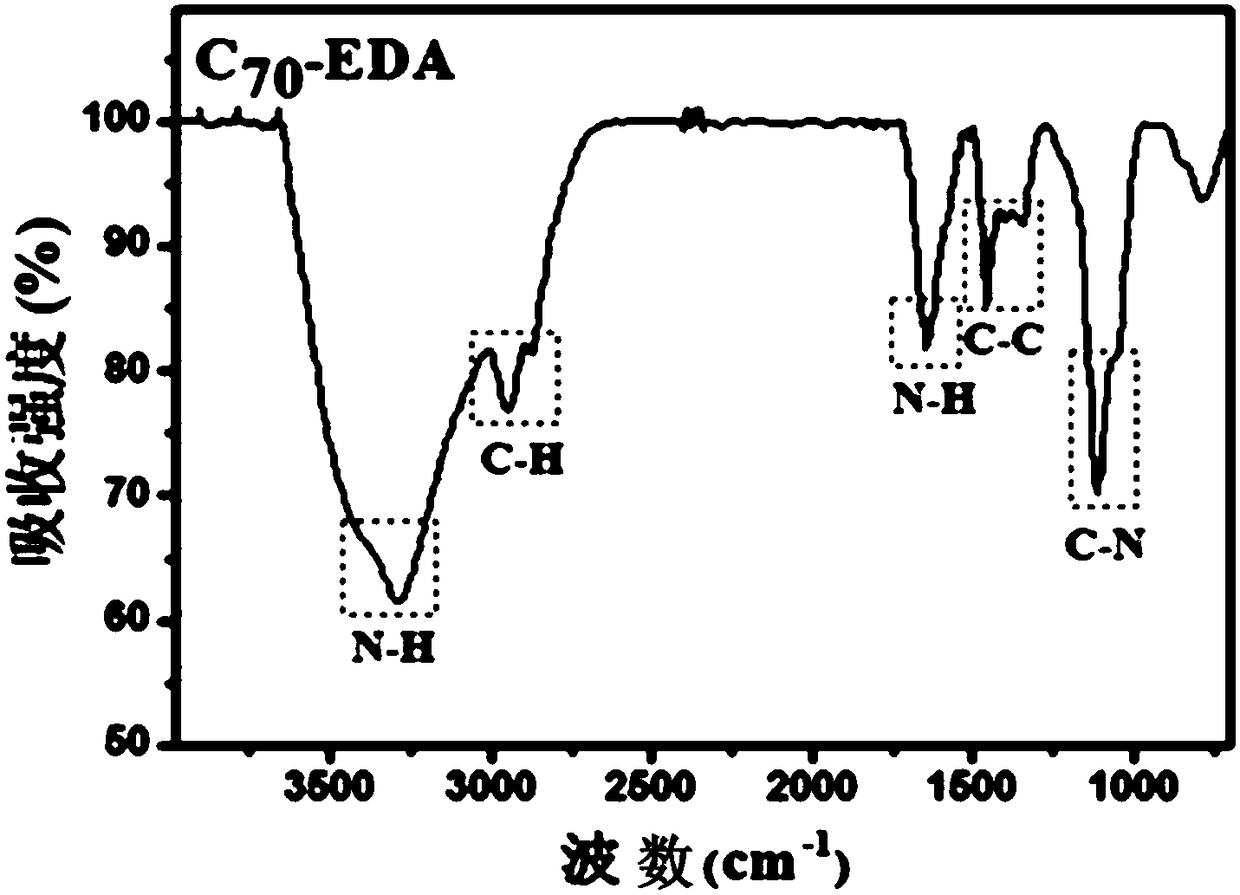
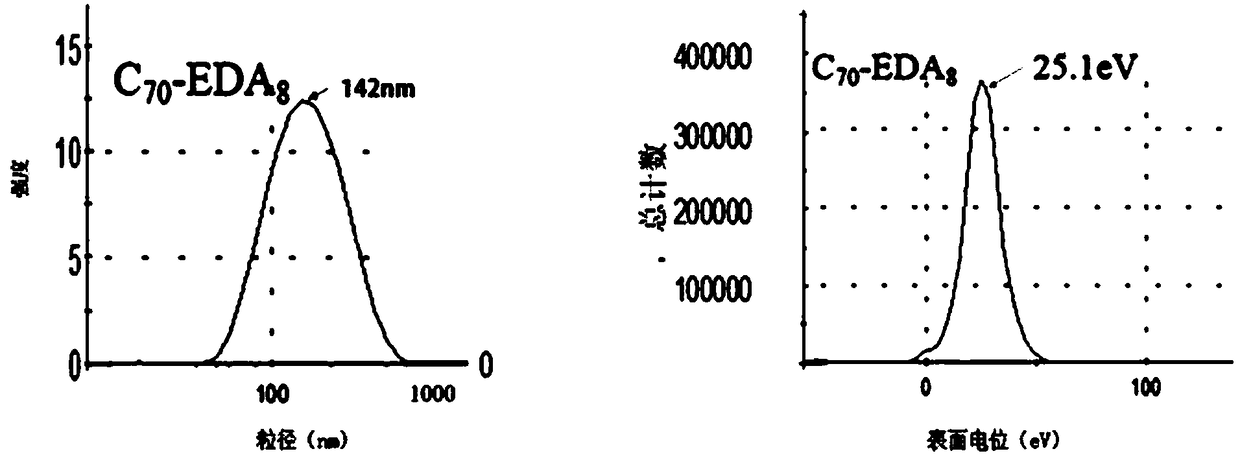
[0050] Example 1: Fullerene ethylenediamine C 70 (EDA) 8 Preparation, infrared spectroscopy detection and dynamic light scattering (DLS) test
[0051] (1) Fullerene ethylenediamine C 70 (EDA) 8 The preparation process
[0052] Fullerene ethylenediamine C 70 (EDA) 8 structural formula
[0053] (a) Weigh 50 mg (ie 0.0595 mol) of solid fullerene C with an analytical balance 70 (purity is 99%, Xiamen Funa New Material Technology Co., Ltd.) was dissolved in 25ml of o-xylene solution, ultrasonically dispersed for 30min, and 50mL (ie 0.75mol) ethylenediamine (analytical grade, national medicine reagent) was measured with a graduated cylinder, Add it into a 100mL Erlenmeyer flask with a stopper with a magnetic stirrer, stir for 24h using a magnetic stirrer at room temperature (rotating speed 1000r / min), use a solvent filter with a volume of 1L and a filter membrane pore size of 200nm (Jinteng Company) The reactant was suction-filtered to obtain a brown-red solution, and the ...
Embodiment 2
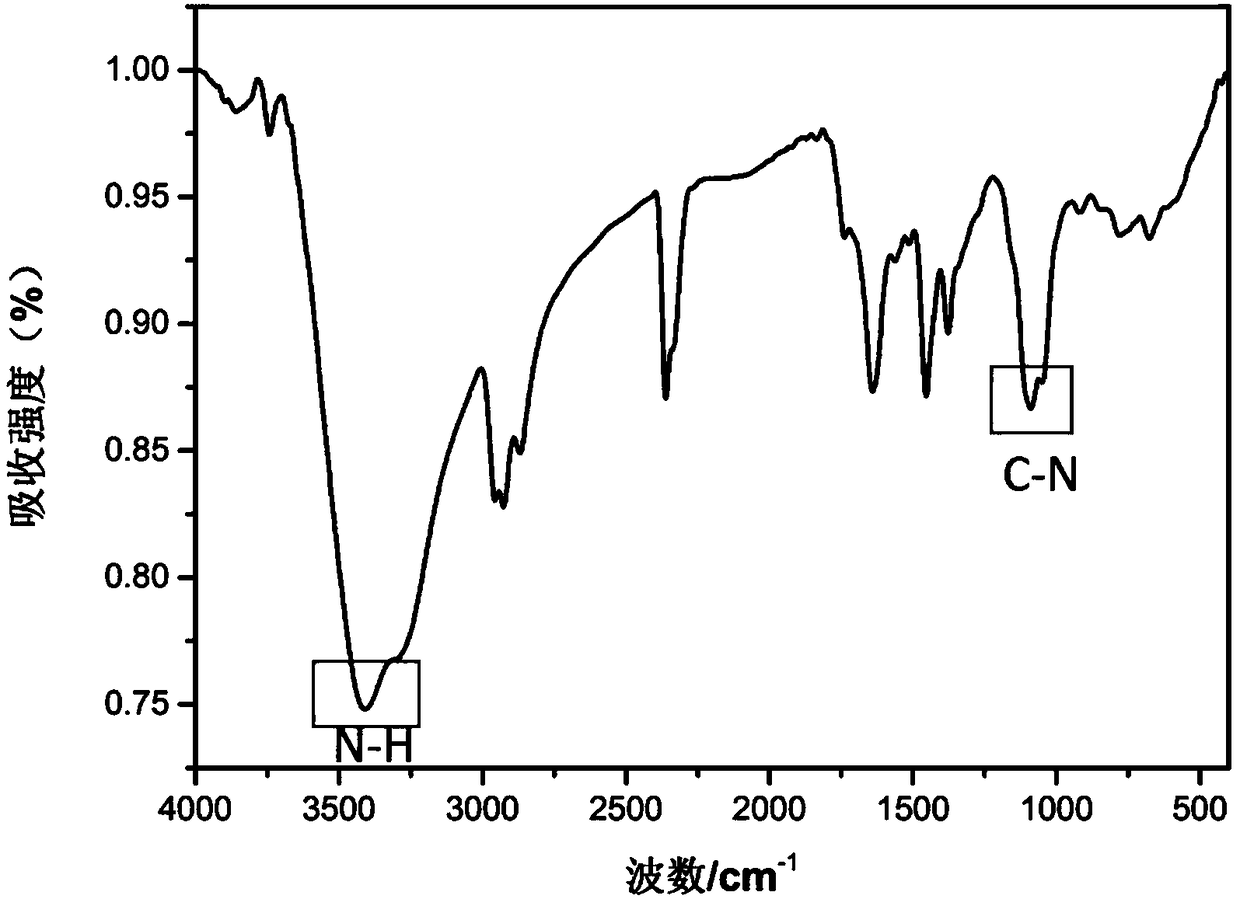
[0063] Example 2: Fuller allyl diamine C 70 (PDA) 6 Preparation, infrared spectroscopy detection and dynamic light scattering test
[0064] (1) Fuller allyl diamine C 70 (PDA) 6 The preparation process
[0065] Fuller allyl diamine C 70 (PDA) 6 structural formula
[0066] (a) Weigh 50 mg (ie 0.0595 mol) of solid fullerene C with an analytical balance 70 (purity is 99%, Xiamen Funa New Material Technology Co., Ltd.) was dissolved in 25ml of o-xylene solution, ultrasonically dispersed for 30min, and 60mL of propylenediamine (i.e. 0.7mol) was measured with a graduated cylinder (analytical grade, Sinopharm Reagent), Add it into a 100mL Erlenmeyer flask with a stopper with a magnetic stirrer, stir for 24h using a magnetic stirrer at room temperature (rotating speed 1000r / min), use a solvent filter with a volume of 1L and a filter membrane pore size of 200nm (Jinteng Company) The reactant was suction-filtered to obtain a brown-red solution, and the components of the soluti...
Embodiment 3
[0075] Example 3: Fullerene butanediamine C 70 (DAB) 2 Preparation, infrared spectroscopy detection and dynamic light scattering test
[0076] (1) Fullerene butanediamine C 70 (DAB) 2 The preparation process
[0077] Fullerene Butanediamine C 70 (DAB) 2 structural formula
[0078] (a) Weigh 50 mg (ie 0.0595 mol) of solid fullerene C with an analytical balance 70 (purity is 99%, Xiamen Funa New Material Technology Co., Ltd.) was dissolved in 25ml of o-xylene solution, ultrasonically dispersed for 30min, and 70mL of butanediamine (ie 0.7mol) was measured with a graduated cylinder (analytical grade, Sinopharm reagent), Add it into a 100mL Erlenmeyer flask with a stopper with a magnetic stirrer, stir for 24h using a magnetic stirrer at room temperature (rotating speed 1000r / min), use a solvent filter with a volume of 1L and a filter membrane pore size of 200nm (Jinteng Company) The reactant was suction-filtered to obtain a brown-red solution, and the components of the so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Hydrated particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com