Gene signature of residual risk following endocrine treatment in early breast cancer
A technology of endocrine therapy and endocrine therapy, applied in the direction of analytical materials, biomaterial analysis, special data processing applications, etc., can solve problems such as accelerating the development of stratification or targeted medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
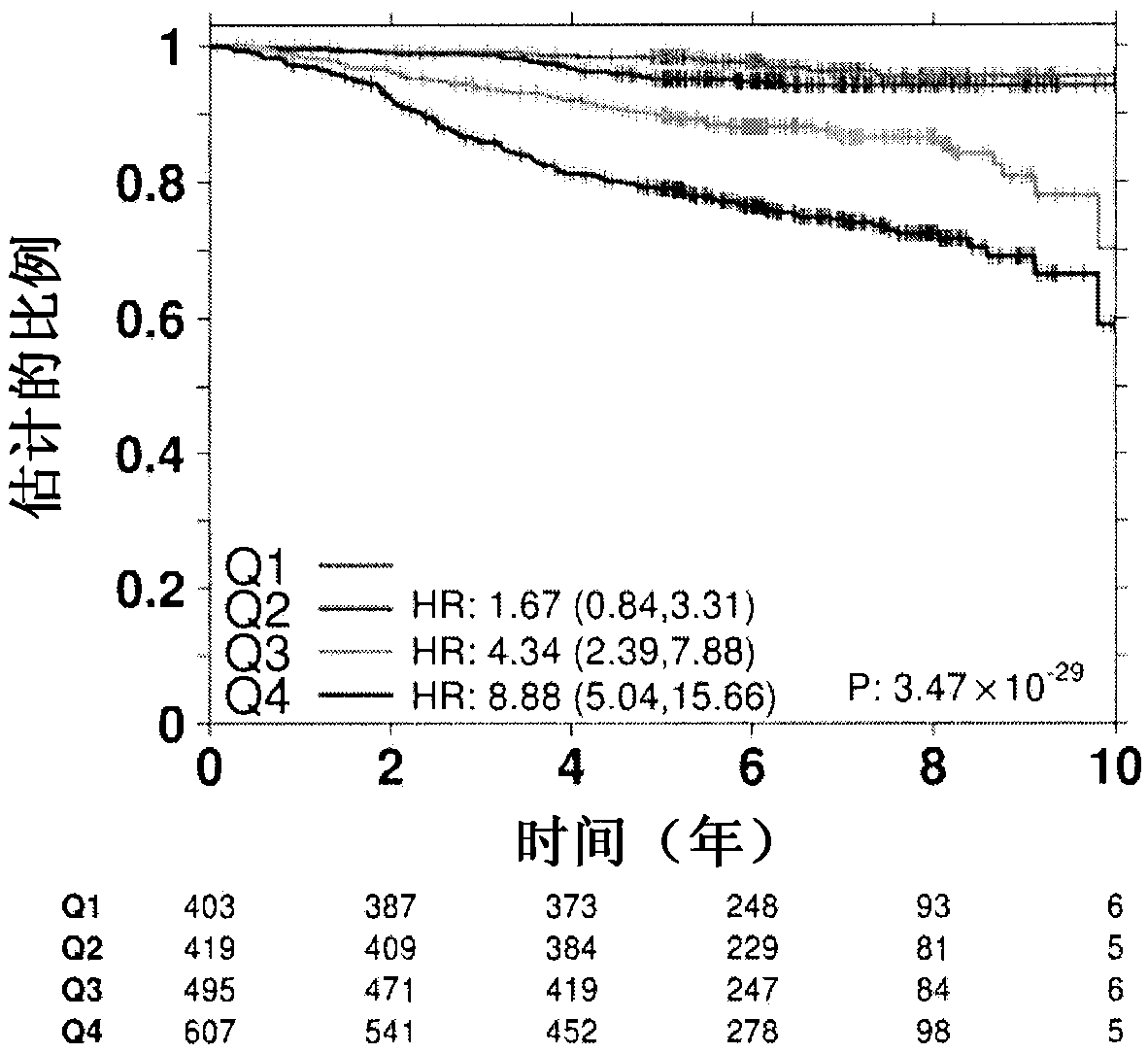
[0101] Materials and methods
[0102] The TEAM trial is a multinational, open-label Phase III trial in which patients with hormone receptor-positive 19 Postmenopausal women with early breast cancer were randomly assigned to receive exemestane (25 mg) or tamoxifen (20 mg) once daily for the first 2.5 to 3 years; exemestane (25 mg) thereafter (total 5 years of treatment) (see Figure 11A ). Hormone-receptor (ER and PgR) and HER2 status assessed locally by immunohistochemistry to enter the trial, followed by central confirmation 28 , and confirmed HER2 status by immunohistochemistry and fluorescence in situ hybridization (FISH) 29 . According to ASCO / CAP guidelines 30-32 , to perform all evaluation work. None of the patients received anti-HER2 therapy. Distant recurrence-free survival (DRFS) was defined as the time from randomization to distant recurrence or death due to breast cancer 19 .
[0103] The TEAM trial included a pathology study consisting of 4,736 patients fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com