Method for preparing difluorochloroacetate and difluorochloroacetic acid through continuous non-catalytic reaction
A technology of difluorochloroacetic acid ester and difluorochloroacetic acid, which is applied in the field of preparation of difluorochloroacetic acid ester and difluorochloroacetic acid, can solve problems that are not conducive to continuous large-scale production, achieve easy separation and purification, and reduce three wastes The production amount and the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
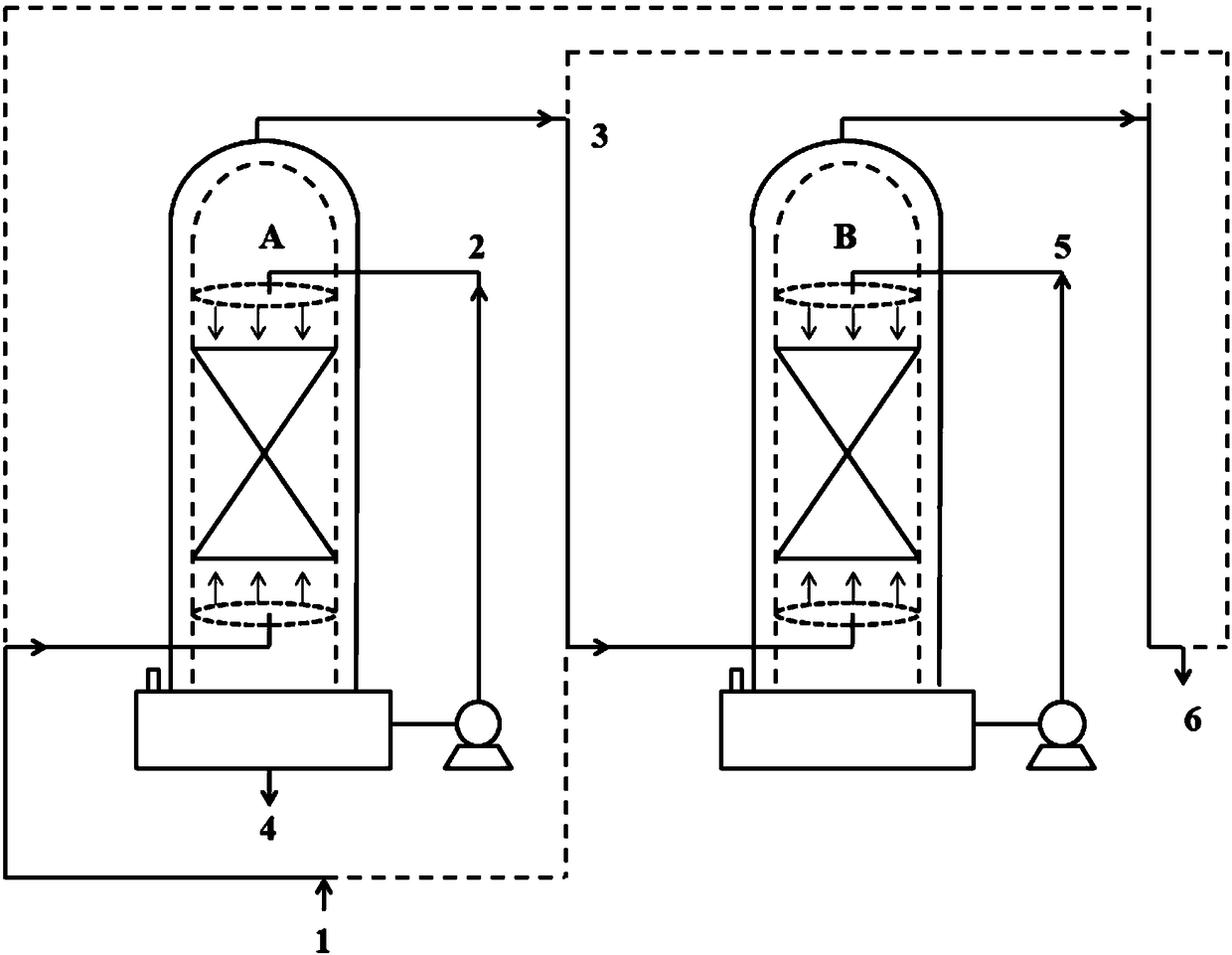
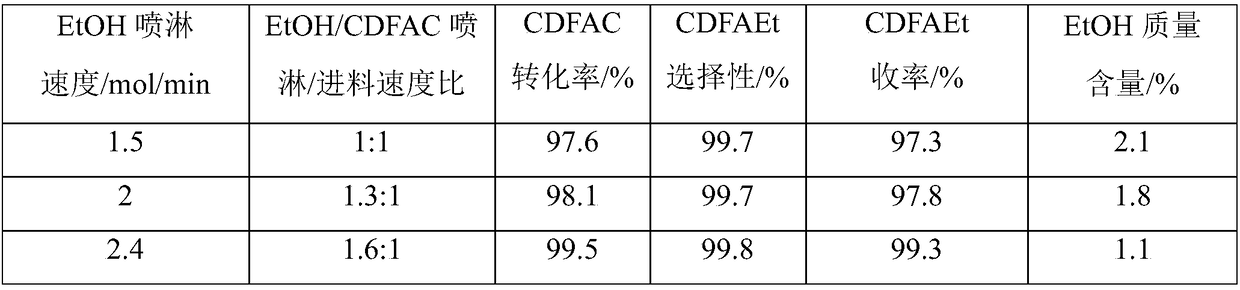
[0045] Such as figure 1 As shown, the jacketed spray towers A and B of 200L enamel materials connected in series are used in two stages, the spray towers A and B are filled with 125L ceramic packing respectively, and the liquid storage areas (100L) of the spray towers A and B are each introduced with 720mol (33170g, 42L) ethanol, wherein spray tower A is a primary spray tower, and spray tower B is a secondary spray tower. Add raw material CClF in spray tower A with the speed of 1.5mol / min (254g / min) 2 COCl stream 1, 90mol (15246g) CClF was added in 1h 2 COCl, add stream 2 ethanol to the spray tower A in a spray manner at an initial speed of 2 mol / min (92 g / min). In the spray tower A, the temperature inside the tower is controlled at about 20°C, and the pressure inside the tower is controlled at about normal pressure, so that the CCIF 2 Reverse contact reaction of COCl and ethanol in the packing area, CClF 2 The residence time of COCl in the packing area is about 3.7min. I...
Embodiment 2
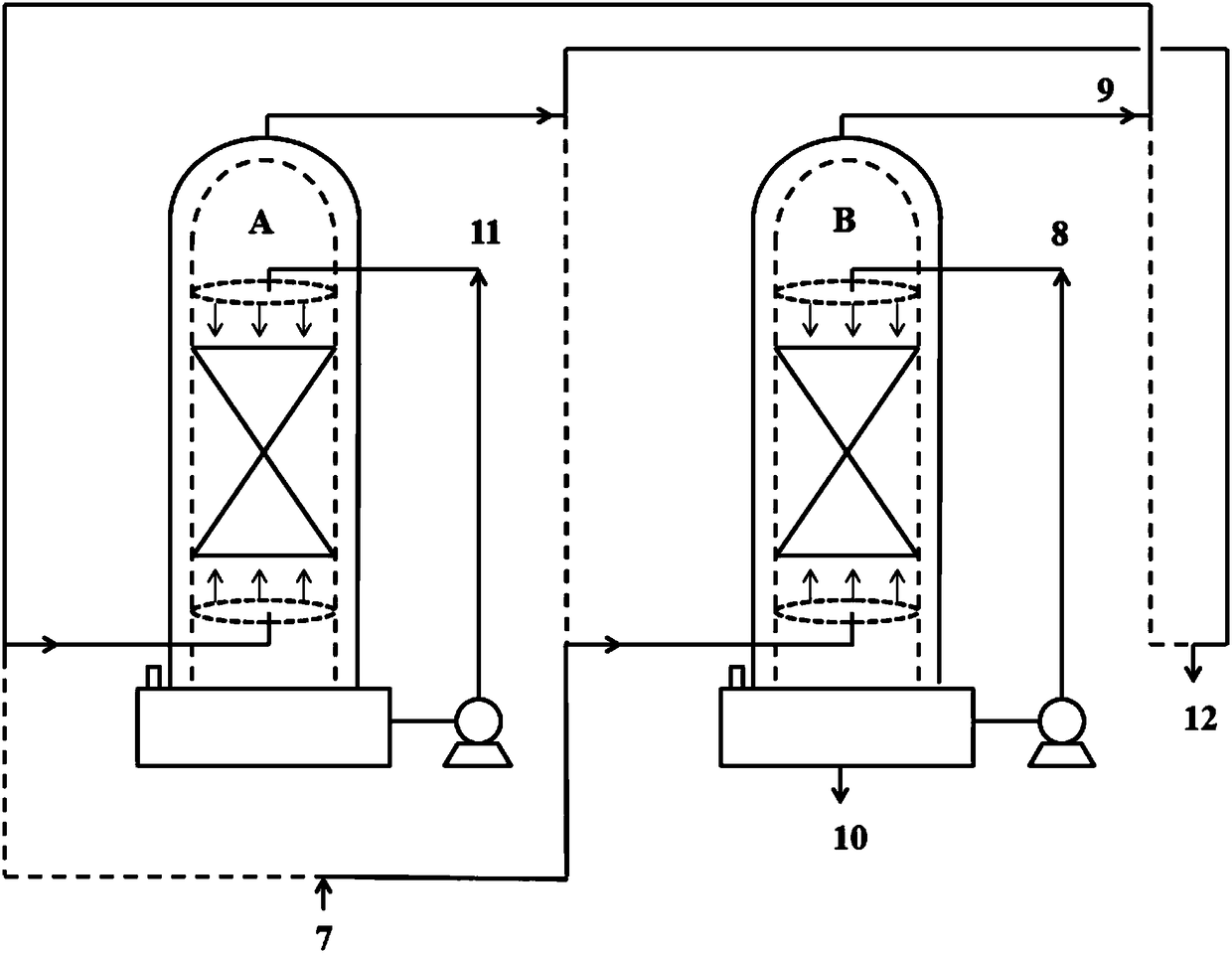
[0048] On the basis of completion of Example 1, the spray tower B that completed the reaction in Example 1 is switched to a primary spray tower, and the spray tower A is switched to a secondary spray tower, and the reaction is continued, such as figure 2 shown.
[0049] Add the raw material CClF to the spray tower B at a speed of 1.5mol / min (254g / min) 2 COCl flow 7, a total of 90mol (15246g) CClF was added in 1h 2 COCl, add stream 8 ethanol to the spray tower B at an initial speed of 2 mol / min (92 g / min) in a spray manner. In the spray tower B, the temperature inside the tower is controlled at about 20°C, and the pressure inside the tower is controlled at about normal pressure, so that the CCIF 2 Reverse contact reaction of COCl and ethanol in the packing area, CClF 2 The residence time of COCl in the packing area is about 3.7min. In the spray tower B, the tower top obtains and contains unreacted CClF 2 The stream 9 of COCl, the stream 9 enters the spray tower A, and the...
Embodiment 3
[0052] On the basis that embodiment 1 and 2 are finished, the spray tower A that finishes reaction among the embodiment 2 is switched to one-stage spray tower, and spray tower B is switched to two-stage spray tower, continues to react, as figure 1 shown.
[0053] Add raw material CClF in spray tower A with the speed of 1.5mol / min (254g / min) 2 COCl stream 1, 90mol (15246g) CClF was added in 1h 2 COCl, add stream 2 ethanol to the spray tower A in a spray manner at an initial speed of 2 mol / min (92 g / min). In the spray tower A, the temperature inside the tower is controlled at about 20°C, and the pressure inside the tower is controlled at about normal pressure, so that the CCIF 2 Reverse contact reaction of COCl and ethanol in the packing area, CClF 2 The residence time of COCl in the packing area is about 3.7min. In the spray tower A, the tower top obtains and contains unreacted CClF 2 Stream 3 of COCl, stream 3 enters spray tower B, and stream 5 ethanol is added to spray t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com