Propellane derivatives based on perylene bisimide C3 symmetry, and synthesis and application thereof
A technology of perylene imide and spirane, which is applied to the spirane derivatives based on perylene imide C3 symmetry and their synthesis and application fields, can solve the problems of low charge conversion efficiency, electronic structure, and aggregated state optoelectronic materials. Performance is less well known and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
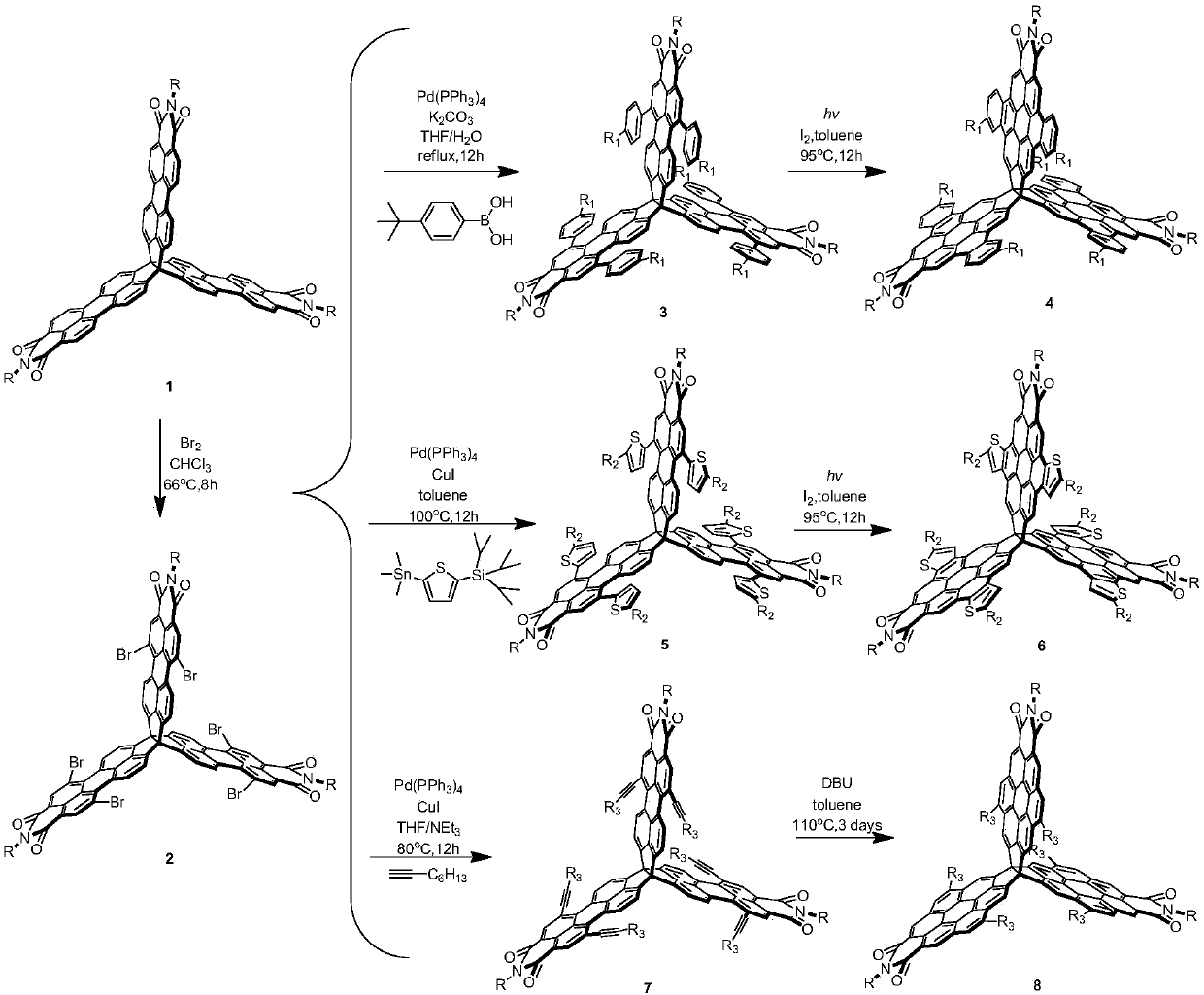
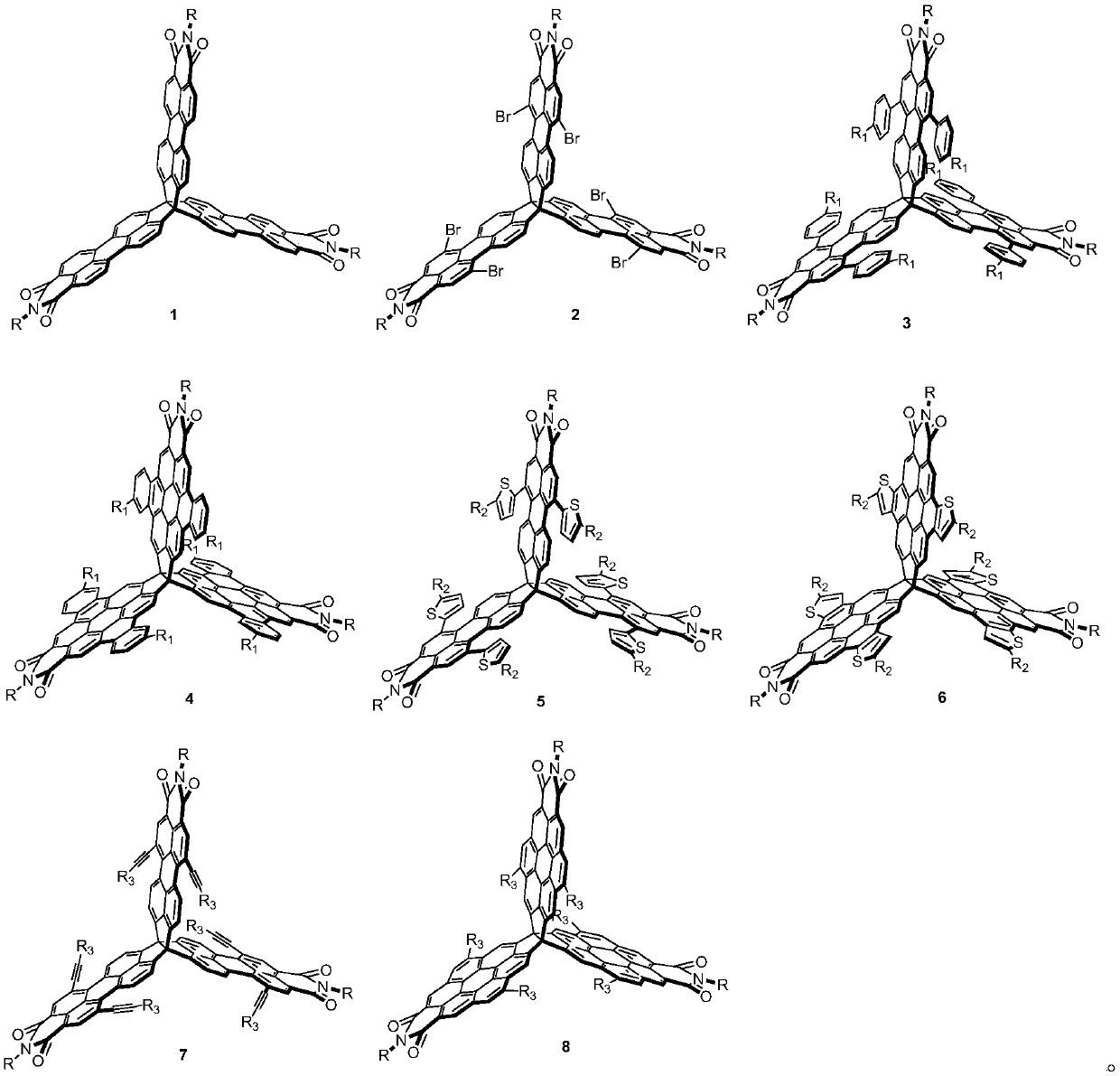
[0043] Synthesis of compound 2a
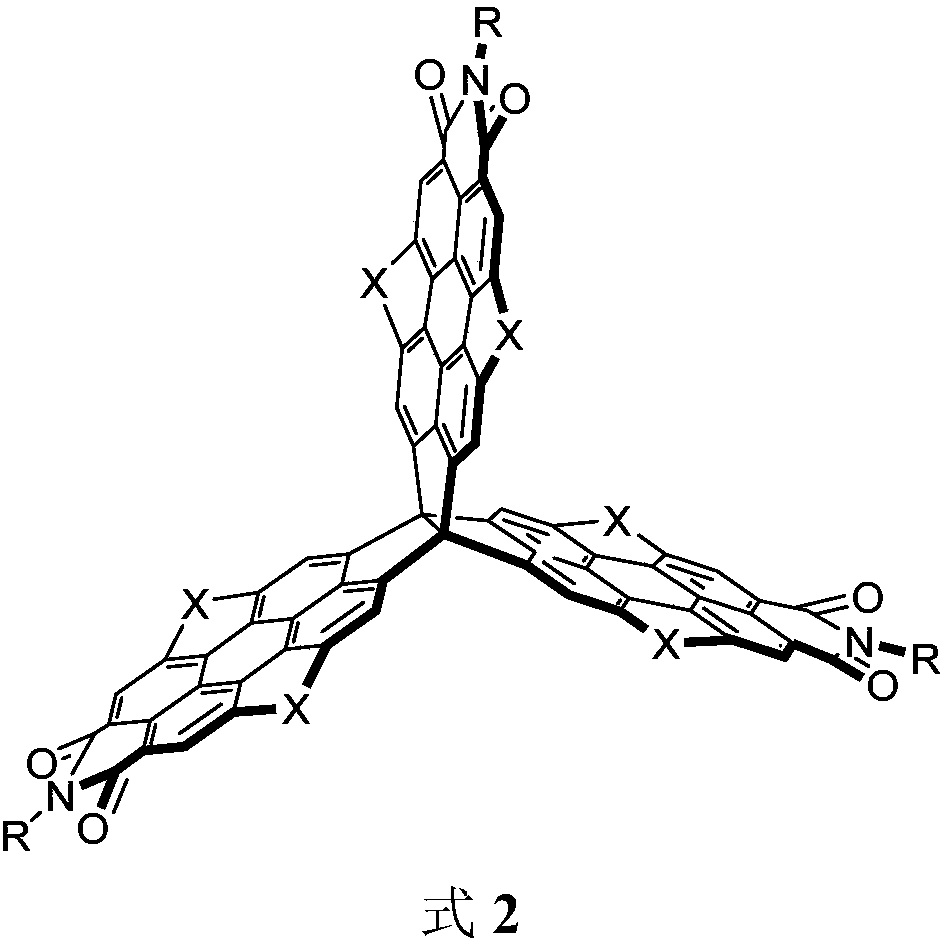
[0044] Compound 1a (100mg, 69.27mmol) and 100mL chloroform solvent were added into a two-necked flask. After heating to reflux, bromine (0.97g, over 120eq.) was added slowly. Reflux for 6 hours, cool, the reaction solution was extracted with saturated aqueous sodium sulfite, and the solvent was removed after drying. The remaining residue was purified and separated on a silica gel column, and the developing solvent was petroleum ether / chloroform (1:1 to 3:1, v / v ) to obtain purple-red compound 2a (60 mg, 45%).
[0045] Bromine is used as a bromination reagent, and the bromination reagent can also be other commonly used bromination reagents, but the effect is different, and the effect of bromine is verified through experiments.
[0046]
[0047] 2a: 1 H NMR (400MHz, CDCl 3 ,300K)δ9.57(d,J=8.0Hz,6H),8.80(s,6H),8.33(d,J=8.0Hz,6H),5.17-5.09(m,3H),2.26-2.16(m ,6H),1.86-1.76(m,6H),1.32-1.20(m,36H),0.81(t,J=6.5Hz,18H);
[0048] 13 C NMR (126...
Embodiment 2
[0062] Synthesis of compound 3a
[0063] Compound 2a (80mg, 0.04mmol), 4-tert-butylphenylboronic acid (9eq., 64.12mg, 0.36mmol) and tetrakis(triphenylphosphine)palladium (30%eq., 13.85mg, 0.01mmol) were added to In a Shrek bottle filled with nitrogen, tetrahydrofuran (20 mL) and 2M potassium carbonate aqueous solution (10 mL) were added sequentially. The reaction solution was heated to 55°C for 12 hours, then the water phase was removed with a separatory funnel to retain the organic phase, the solvent was evaporated to dryness under reduced pressure, the filter residue was washed with methanol and dried. The dried crude product was further purified using a silica gel column to obtain the purple product 3a (53 mg, 59%), and the entire post-treatment process needs to be protected from light.
[0064]
[0065] 3a: 1 H NMR (500MHz, CDCl 2 CDCl 2 ,373K)δ=8.41(s,6H),7.80(d,J=8.1Hz,6H),7.56(d,J=8.1Hz,12H),7.40(dd,J=7.8,5.4Hz,18H), 5.18-5.14(m,3H),2.28-2.21(m,6H),1.93-1.88(m,6...
Embodiment 3
[0069] Synthesis of Compound 4a
[0070] In a standard photochemical reaction tube, compound 3a (2×40 mg, 35.77 mmol), toluene (2×40 mL) and iodine (5 mg) were added. The reaction solution was irradiated under blue light (460-465 nm) at 95° C. for 12 hours. Then, the reaction solution was extracted with saturated aqueous sodium sulfite solution, and then the aqueous phase was removed with a separatory funnel to retain the organic phase. The solvent was evaporated to dryness under reduced pressure, and the filter residue was washed with methanol and dried. The dried crude product was further purified by silica gel column (PE:DCM=2:1, v:v) to obtain yellow compound 4a (60 mg, 75%).
[0071]
[0072] 4a: 1 H NMR (400MHz, CDCl 3 ,300K)δ=10.90(s,6H),10.54(d,J=15.1Hz,6H),9.96(s,6H),9.53(d,J=8.8Hz,6H),8.32(d,J=8.7 Hz,6H),5.49-5.42(m,3H),2.47-2.37(m,6H),2.14-1.87(m,60H),1.34-1.18(m,36H),0.83-0.74(m,18H);
[0073] 13 C NMR (125MHz, CDCl 2 CDCl 2 ,373K):δ=166.38,152.24,147.07...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com