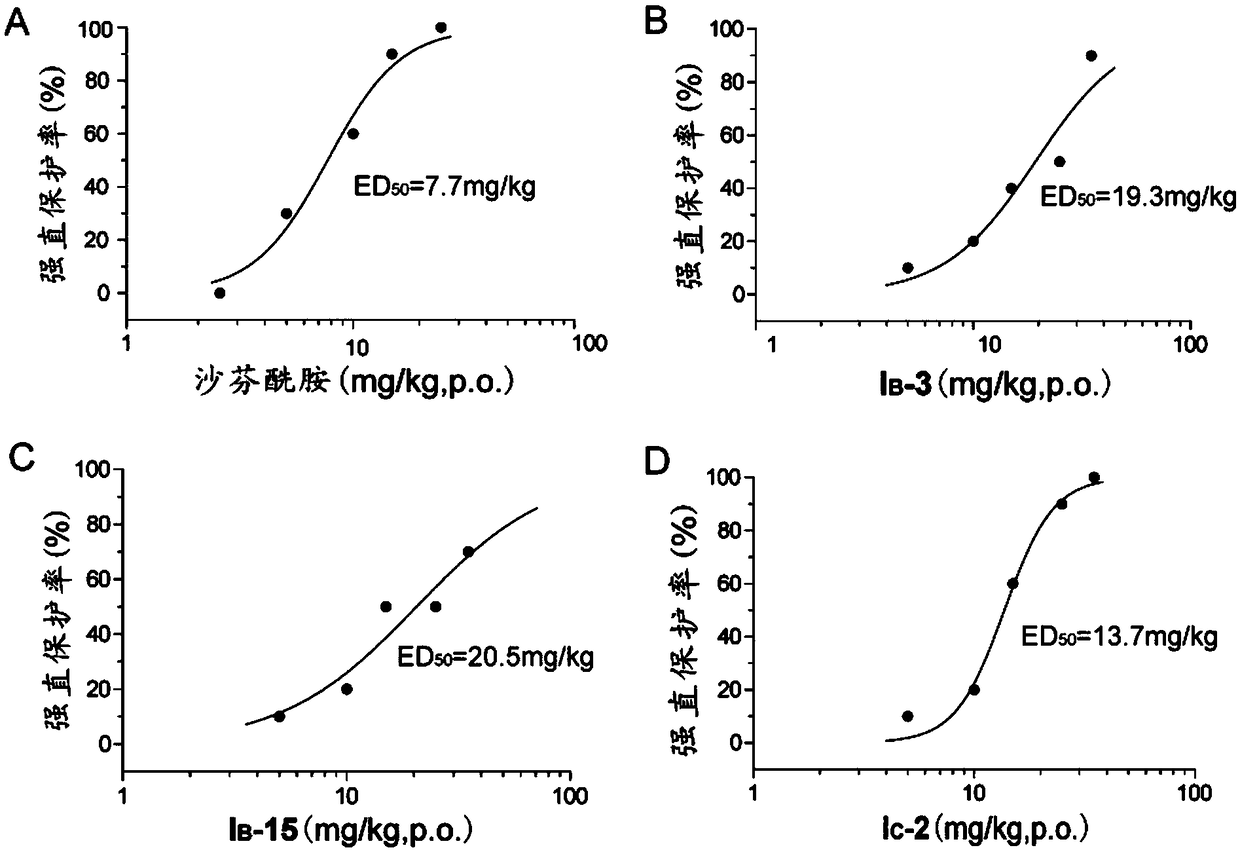
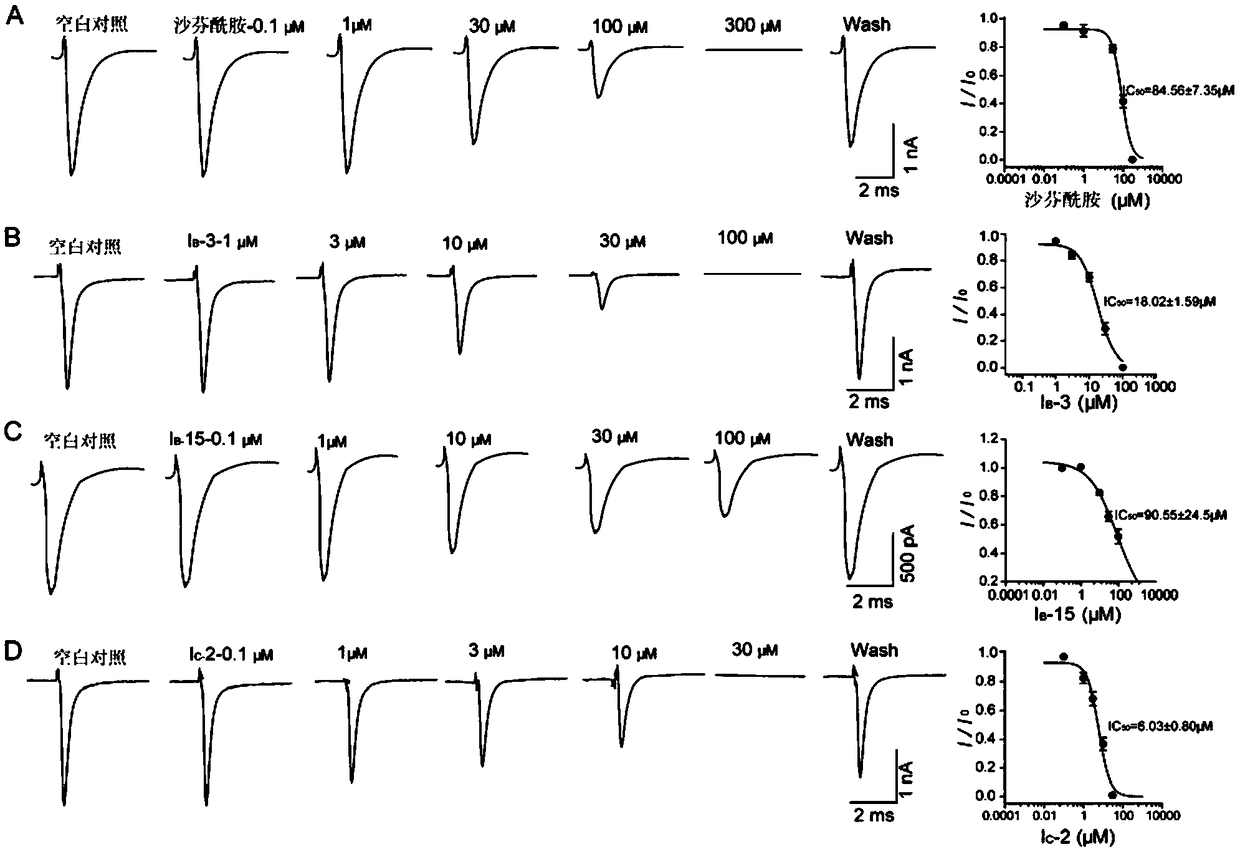
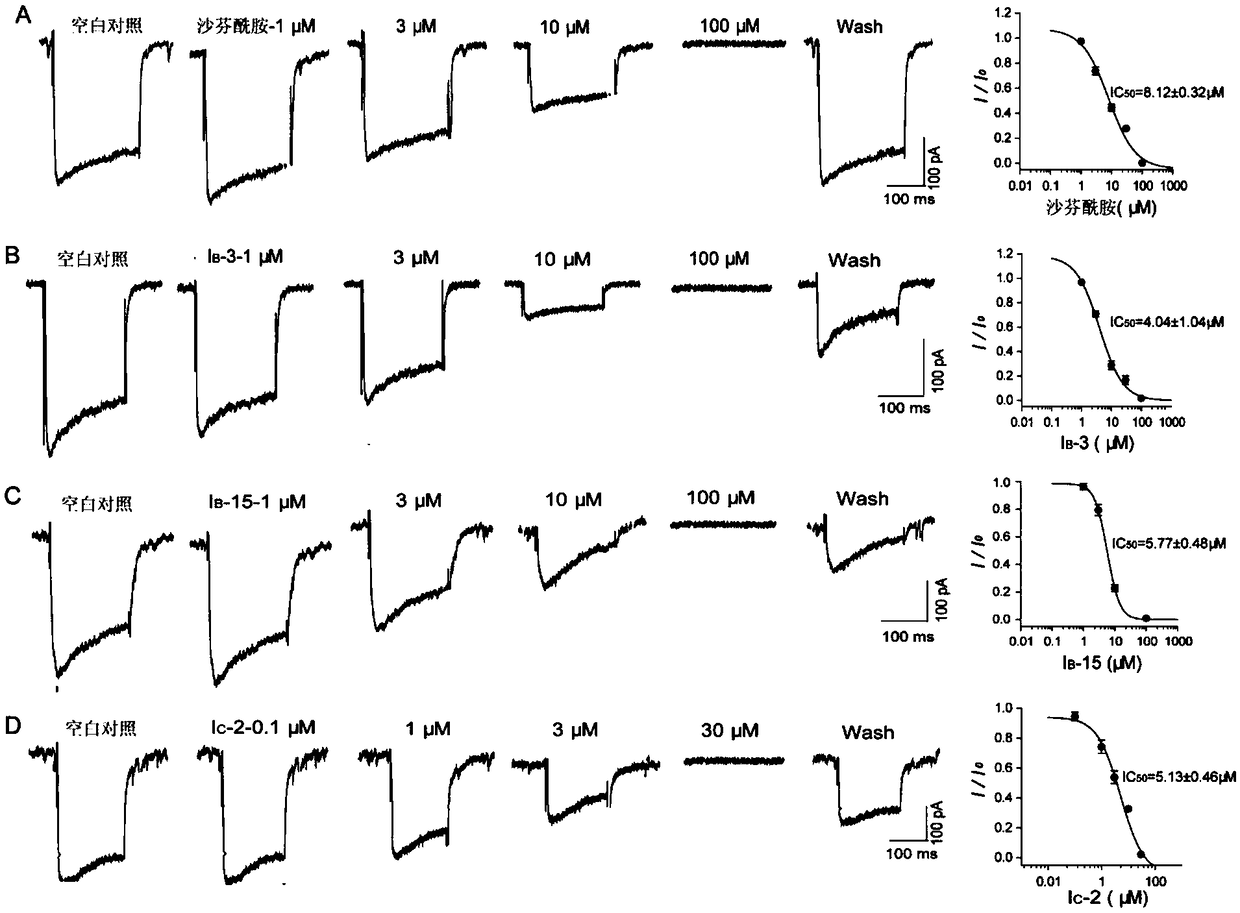
Aminoacetamide compound containing benzo-aliphatic ring structure and usage thereof
A technology of aminoacetamide and compounds, applied in the field of medicinal chemistry and pharmacotherapeutics, can solve serious and ineffective control of epileptic seizures, side effects and other problems, and achieve good anticonvulsant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] The method for the compound shown in the preparation formula I provided by the invention specifically comprises the following steps:
[0062] Starting from dihydrocoumarin
[0063] (1) Stir the mixture of aluminum chloride, sodium chloride and dihydrocoumarin at 200°C to 210°C for 1 hour to 2 hours, add it to ice water, then add concentrated hydrochloric acid to make the reaction system acidic, After filtering, the filter cake was washed with ethanol and dried to obtain intermediate II (4-hydroxyl-1-indanone);
[0064] (2) Dissolve intermediate II, sodium cyanoborohydride, and zinc iodide in 1,2-dichloroethane, heat to reflux for 4 to 6 hours, filter while hot, collect the filtrate and concentrate, and the residue is passed through Separation by column chromatography to obtain intermediate III (4-indenol);
[0065] (3) Dissolve the intermediate III in dichloromethane, then add tin tetrachloride while stirring under the protection of nitrogen at 0°C, after the system...
Embodiment 1
[0095] (1) Preparation of 4-hydroxyl-1-indanone (intermediate II):
[0096] Stir the mixture of 20 grams of aluminum chloride, 4 grams of sodium chloride and 4 grams of dihydrocoumarin at 200 ° C ~ 210 ° C for 1.5 hours, cool to room temperature, pour the system into an appropriate amount of ice water, add 30 ml concentrated Hydrochloric acid made the system strongly acidic, then suction-filtered with a triangular funnel, retained the filter cake, washed the filter cake with 20 ml of ethanol, and finally dried the filter cake to obtain the title compound (intermediate II), 3.24 g of a brown solid, and rate of 81%.
[0097] 1 H-NMR (400MHz, DMSO-d 6 )δ9.94(s,1H),7.24(t,J=7.6Hz,1H),7.09(d,J=7.4Hz,1H),7.04(d,J=7.8Hz,1H),2.91(t, J=5.8Hz, 2H), 2.60(t, J=5.8Hz, 2H).
[0098] (2) Preparation of 4-indanol (intermediate III):
[0099] Dissolve 2 grams of intermediate II, 2.6 grams of sodium cyanoborohydride, and 13 grams of zinc iodide in 50 milliliters of 1,2-dichloroethane, heat...
Embodiment 2
[0105] (1) 7-(3-fluorobenzyloxy)-2,3-dihydro-1-H-indene-4-carbaldehyde (compound shown in formula V-1, abbreviated as "intermediate V-1", hereinafter the same) preparation:
[0106]
[0107] Add 120 mg of intermediate IV, 107 μl of 3-fluorobenzyl chloride, and 255 mg of potassium carbonate into 3 ml of N,N-dimethylformamide, react at 90°C for 4 hours, cool the system to room temperature, and add Add water and ethyl acetate, extract the aqueous layer twice with ethyl acetate, collect the organic layer, wash the organic layer twice with water, wash once with saturated sodium chloride, add anhydrous magnesium sulfate to dry, filter, concentrate, and the residue is After separation by column chromatography, the title compound (Intermediate V-1) was obtained as a yellow solid (120 mg) with a yield of 65%.
[0108] 1H-NMR (400MHz, CDCl 3 )δ10.02(s,1H),7.63(d,J=7.7Hz,1H),7.42-7.31(m,1H),7.17(dd,J=17.2,8.4Hz,2H),7.03(t,J =8.2Hz, 1H), 6.81(d, J=7.6Hz, 1H), 5.18(s, 2H), 3.32(t, J...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com