Aluminum sol production method and aluminum sol production system
A production method, aluminum sol technology, applied in sol preparation, chemical instruments and methods, alumina/aluminum hydroxide, etc., can solve the problems of low purity of aluminum sol, achieve low impurity content, mild conditions, and low production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
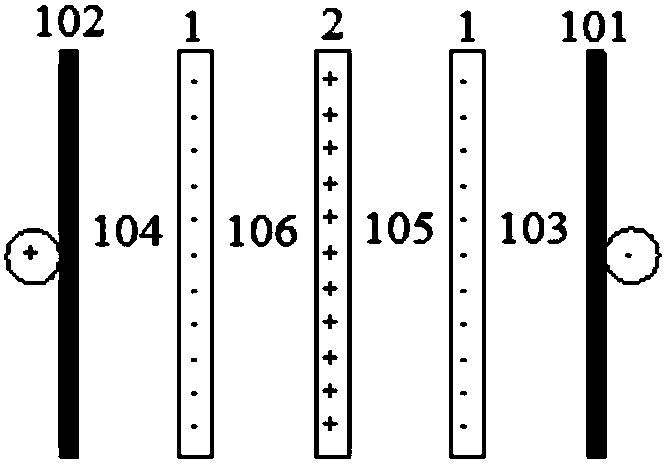
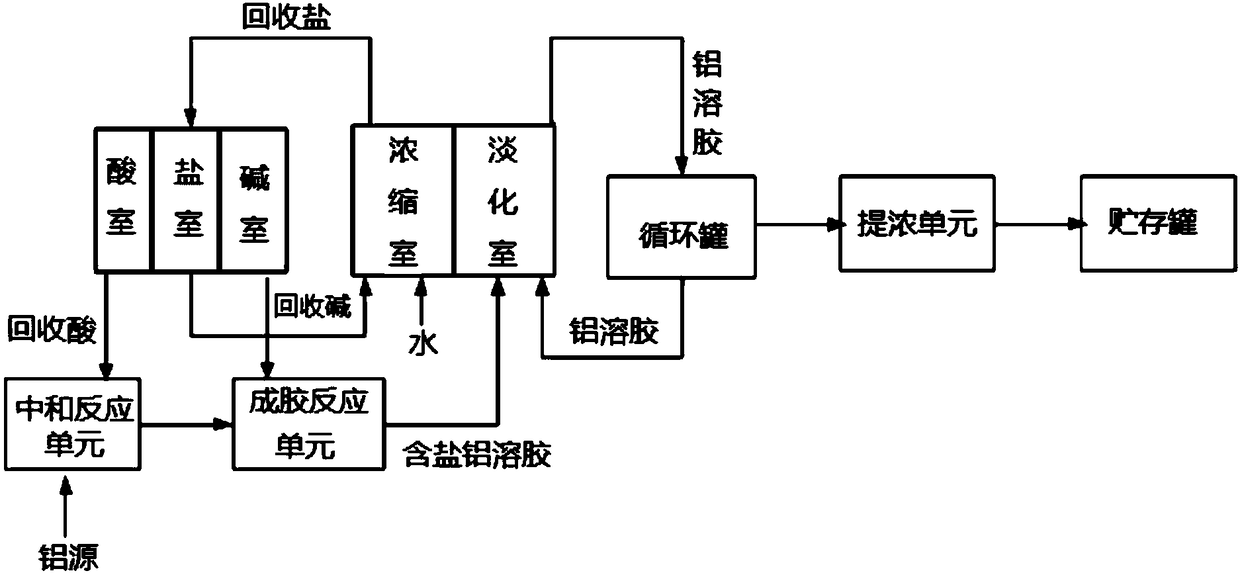
[0102] This embodiment adopts image 3 The production system shown, the specific operation process is as follows.
[0103] (1) Neutralization reaction
[0104] Add industrial hydrochloric acid with a concentration of 32% by weight into the neutralization reactor, and add water to dilute to a concentration of 20% by weight at ambient temperature (25° C., the same below). Then, aluminum hydroxide powder was added, and the temperature of the reactor was raised to 95° C. with stirring (stirring rate was 350 rpm), and after constant temperature for 3 hours, the temperature of the reactor was lowered to ambient temperature. The reaction solution was filtered to obtain an aluminum chloride solution having a concentration of 20% by weight. Among them, the molar ratio of aluminum hydroxide to HCl is 1:3.2.
[0105] (2) Gelling reaction
[0106] With stirring, 816 g of a 15% strength by weight aqueous solution of sodium hydroxide were slowly added to 877 g of the aluminum chloride s...
Embodiment 2
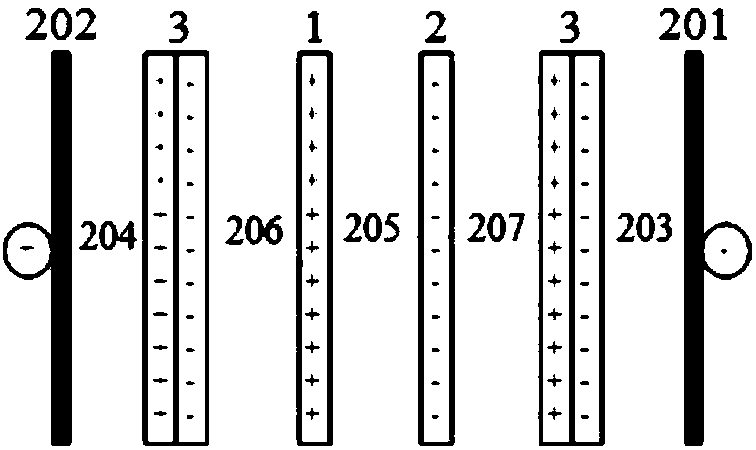
[0115] Adopt the same method as Example 1 to carry out ordinary electrodialysis, the difference is that the electrolyte content in the electrolyte in the cathode chamber of the ordinary electrodialyzer is 3% by weight, and the electrolyte content in the electrolyte in the anode chamber is Content is identical with embodiment 1. As a result, after the ordinary electrodialyzer was operated under this condition for 8 hours, the internal resistance increased, and normal electrodialysis could not be performed. It was found that the surface of the cation exchange membrane adjacent to the cathode was severely scaled, and the cation adjacent to the cathode was replaced. After the membrane is exchanged, the electrodialyzer resumes normal operation.
Embodiment 3
[0117] Ordinary electrodialysis was carried out in the same manner as in Example 1, except that the electrolyte content in the electrolyte in the cathode chamber and anode chamber of the ordinary electrodialyzer was 3% by weight. As a result, the efficiency of ordinary electrodialysis decreased after 8 hours of operation under this condition. It was found that the surface of the cation exchange membrane adjacent to the cathode was scaled after shutdown for maintenance. After replacing the cation exchange membrane adjacent to the cathode, the electrodialyzer resumed normal operation. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com