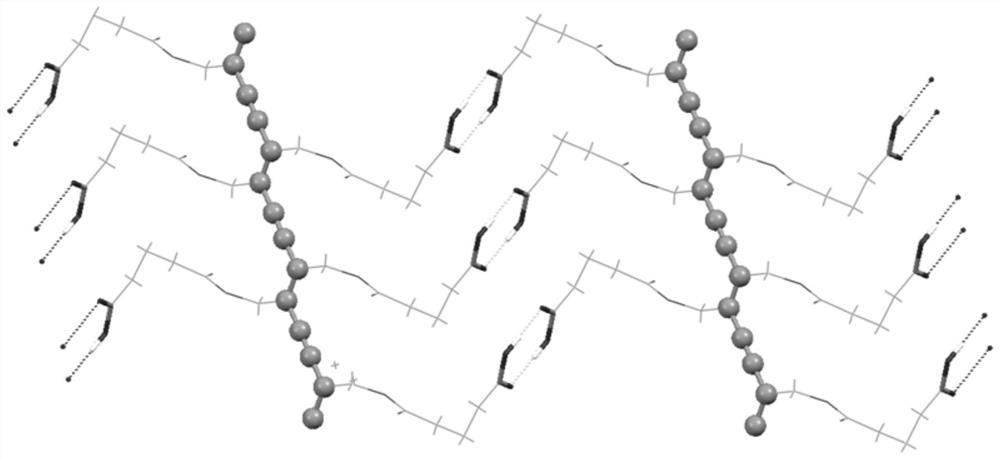
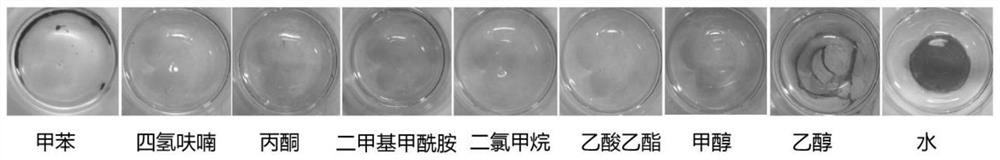
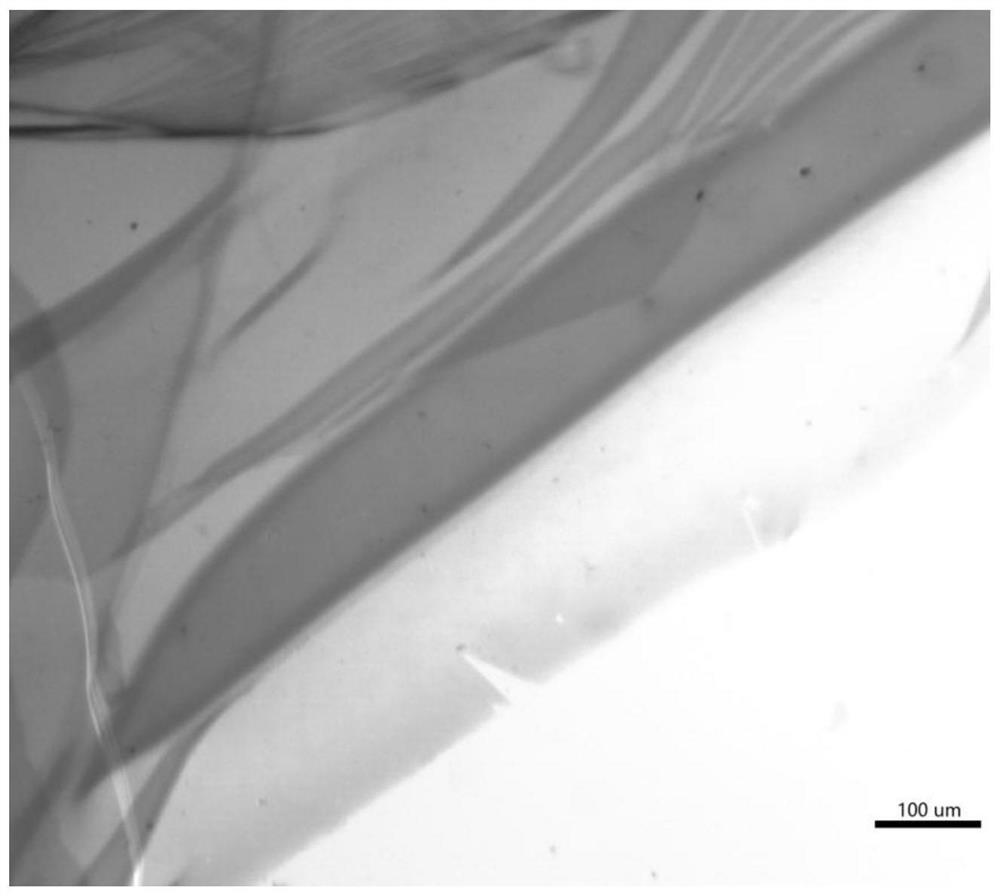
A diyne monomer and its preparation method, a polydiyne and its preparation method and application
A bidiyne and monomer technology, applied in the field of functional materials, can solve the problems of complex electrospinning film forming process, little difference in color change, narrow application range, etc., achieve low synthesis cost, simple and easy control of synthesis conditions, large-scale effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1. Synthesis of Diyne Monomer:
[0044] Add 5.5g of 2,4-hexadiyn-1,6-diol, 12.0g of glutaric anhydride, 0.05g of 4-dimethylaminopyridine (DMAP) and 20mL of dry tetrahydrofuran into the reaction vessel, and stir Heated to 50°C, kept the temperature constant and continued to stir for 10 hours. After the reaction was completed, the tetrahydrofuran was removed by rotary evaporation, and the residue was repeatedly washed with deionized water for 3 times, and then purified by silica gel column (ethyl acetate as eluent). , to obtain 13.5 g light yellow solid, the yield is 80%.
[0045] Melting point: 6 , 600MHz) δ / ppm: 12.11 (s, 2H, COOH); 4.84 (s, 4H, OCH 2 ); 2.39(t,J=7.5Hz,4H,CH 2 COO); 2.25(t, J=7.5Hz, 4H, CH 2 COO); 1.74(m, J=7.65Hz, 4H, CH 2 ); 13 C NMR (DMSO-d 6 , 150MHz) δ / ppm: 173.88; 171.77; 75.18; 69.12; 51.84; 32.45; 32.19; 19.73; 16 h 18 o 8 [M-H] - 337.0929.
[0046] As can be seen from the above identification and analysis results, the light yellow so...
Embodiment 2
[0056] 1. Synthesis of Diyne Monomer:
[0057] Add 1.1g of 2,4-hexadiyne-1,6-diol, 2.4g of succinic anhydride, 0.05g of DMAP and 10mL of dry tetrahydrofuran into the reaction vessel, and heat to 60°C while stirring, keeping the temperature constant After stirring for 5 hours, the tetrahydrofuran was removed by rotary evaporation, and the residue was repeatedly washed with deionized water for 3 times, and then purified by silica gel column (ethyl acetate as eluent) to obtain 2.9 g of light yellow solid , which is a monomer, and the yield is 93%.
[0058] Melting point: 135-137°C; 1 H NMR (MeOD, 600MHz)δ / ppm: 4.79(s,4H,OCH 2 ); 2.62(m,8H,CH 2 ); 13 C NMR (MeOD, 150MHz) δ / ppm: 175.75; 173.18; 74.92; 70.53; 53.11; 29.71; 29.57; 16 h 18 o 8 [M-H] - 309.0616.
[0059] As can be seen from the above-mentioned identification and analysis results, the light yellow solid obtained is bis-diacetylene di-succinate, and its structural formula is as follows:
[0060]
[0061] 2. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com