Method for rapidly dissolving/swelling water-soluble high polymer suspending agent in drug suspension
A technology of water-soluble polymers and suspending agents, which can be used in pharmaceutical formulations, organic active ingredients, liquid transportation, etc., and can solve problems such as the physical stability of suspensions, and achieve the effects of saving preparation time and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
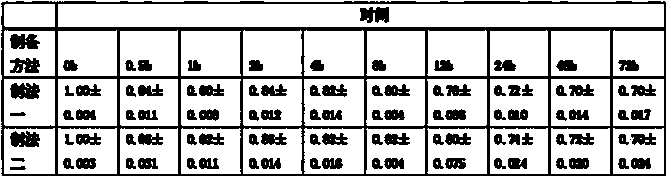
Image
Examples
Embodiment 1
[0012] Example 1 Preparation of Sulfadiazine Suspension
[0013] Prescription: Sulfadiazine 10.0g
[0014] Propylene glycol 15.0ml
[0015] Ethylparaben 0.004g
[0016] Butylparaben 0.001g
[0017] Sodium carboxymethyl cellulose 1.0~1.2g
[0018] Add purified water to 100ml
[0019] Preparation method: Weigh 0.004g of ethylparaben and 0.001g of butylparaben, stir and dissolve them in 15.0ml of propylene glycol to obtain solution A; weigh 1.0g of sodium carboxymethylcellulose, and stir Add it into solution A, stir and mix well to get mixed solution B; accurately weigh 10.0g of qualified sulfadiazine raw material, add it to about 70ml of water, stir it evenly to get mixed solution C; slowly add mixed solution B to mix In solution C, stir and mix evenly while adding, and finally add purified water to make up to 100ml to get ready. After passing the inspection, subpackage, cover, label, pack, inspect, and store.
Embodiment 2
[0020] Example 2 Preparation of Enrofloxacin Suspension Injection
[0021] Prescription: Enrofloxacin 10.0g
[0022] EDTA-2Na 0.01g
[0023] Polyethylene glycol 10ml
[0024] NaHSO 3 0.2g
[0025] Ethylparaben 0.1g
[0026] Butylparaben 0.025g
[0027] Hydroxyethylcellulose 0.5g
[0028] Sodium citrate 0.2g
[0029] Add water for injection to 100ml
[0030] Preparation method: Weigh the prescribed amount of ethylparaben and butylparaben, dissolve them in 10ml of polyethylene glycol to obtain solution A; weigh the prescribed amount of hydroxyethyl cellulose, add it to solution A, and Stir while adding until evenly dispersed to obtain mixed liquid B; weigh the prescribed amount of EDTA-2Na, NaHSO 3 , enrofloxacin, dissolved / dispersed in about 70ml of water for injection in sequence to obtain mixed solution C; slowly add mixed solution B to mixed solution C, stirring while adding, after fully mixing, add the prescribed amount of lemon Sodium acid, stir and mix, add wat...
Embodiment 3
[0031] Example 3 Preparation of Albendazole Suspension
[0032] Prescription: Albendazole 20g
[0033] Propylene glycol 20ml
[0034] Sodium Carboxymethyl Cellulose 1.0g
[0035] Methylparaben 0.30g
[0036] Add purified water to 200ml
[0037] Preparation:
[0038] Preparation method 1: Take about 100ml of purified water, sprinkle the prescribed amount of sodium carboxymethyl cellulose in the water, stir and mix, and let it stand for about 10 hours until it is fully dissolved to obtain solution A; add the prescribed amount of Albenda Add azole to about 70ml of purified water, stir and mix to obtain mixed solution B; dissolve 0.30g of methylparaben in 20ml of propylene glycol to obtain solution C; add B and C to solution A in turn, and stir while adding Mix well, add purified water to make up to full volume, and you get it.
[0039] Method 2: Weigh the prescribed amount of methylparaben and sodium carboxymethylcellulose, first dissolve methylparaben in 20ml of propylene ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com