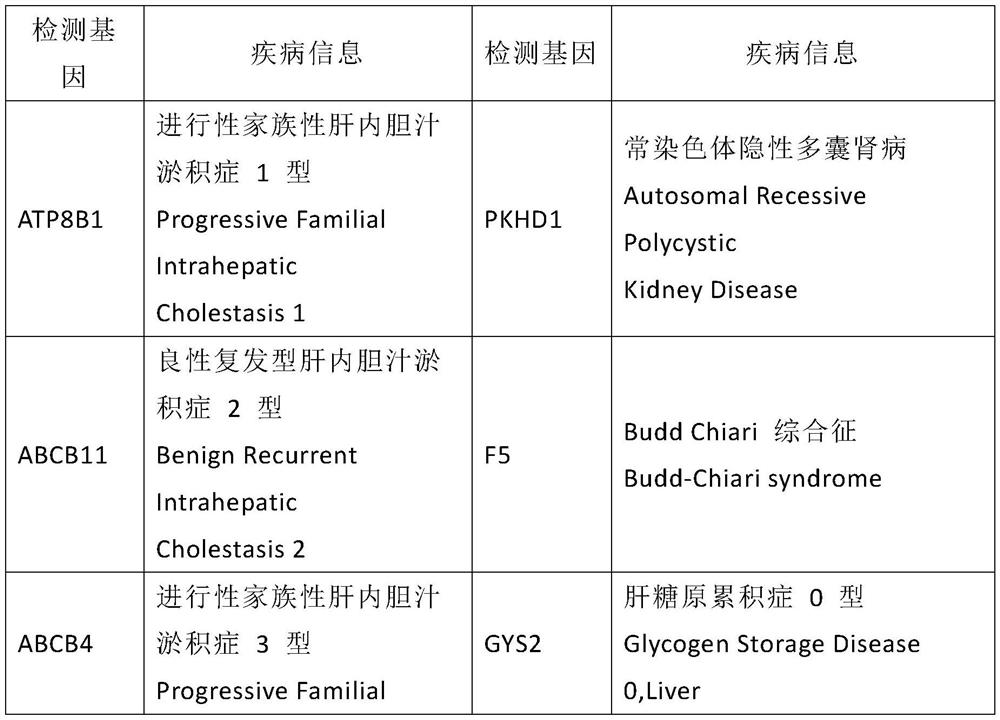
A kit for screening genetic liver diseases
A hereditary and kit technology, which is applied in the field of hereditary liver disease genetic detection kits, can solve the problems of poor repeatability, unsatisfactory detection of hereditary liver diseases, the increase of detectable diseases year by year, false positives and false negatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Detection of Gene Mutations in Inherited Liver Diseases
[0035] 1. The clinical blood samples involved in this experiment came from the Affiliated Hospital of Hangzhou Normal University. Immediately after sample collection, store in a -80°C refrigerator.
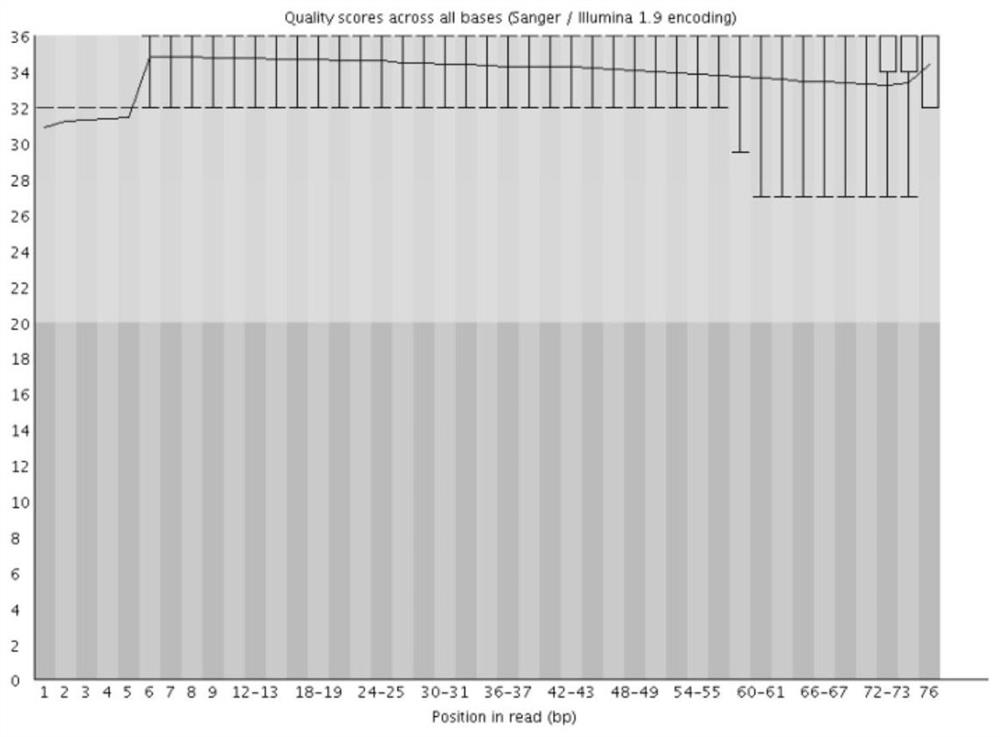
[0036] 2. All reagents are purchased from regular manufacturers. The purity of all primers reached electrophoresis grade (PAGE) or HPLC grade, without any bands.
[0037] 3. Genomic DNA extraction
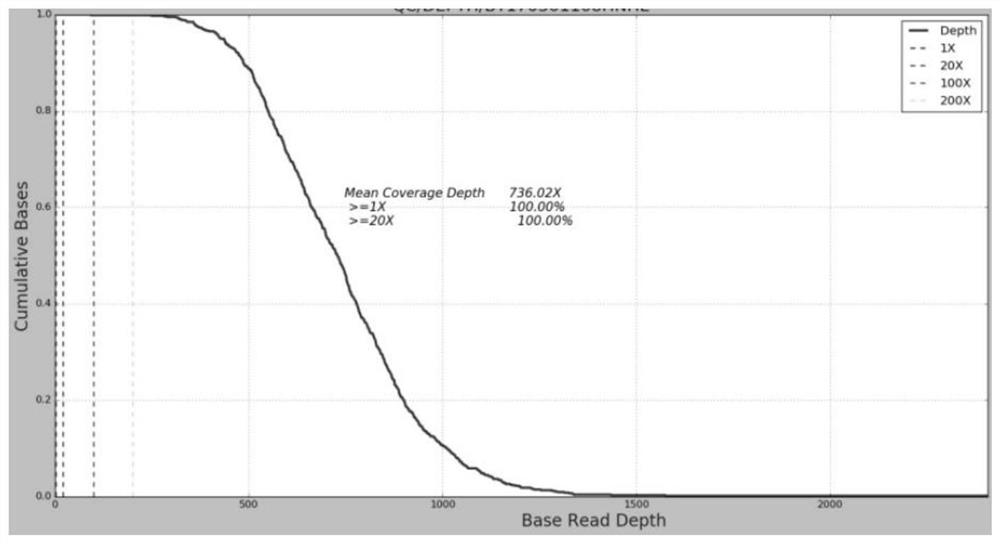
[0038] After mixing the whole blood, DNA was extracted using QIAamp DNA Blood Mini kit (Qiagen, USA). DNA concentration was quantified using Qubit dsDNA HS assay kit and Qubit Fluorometer (Life Technologies, USA). DNA purity (A260 / 280 and A260 / 230) was identified using Nanodrop-2000 (Thermo Fisher Scientific, USA).
[0039] 4. Library Preparation
[0040] Genomic DNA was fragmented into 350 or 550bp fragments using Covaris M220 (Covaris, USA). The sequencing library was prepared by Accel-NGS 2S Hyb DNA L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com