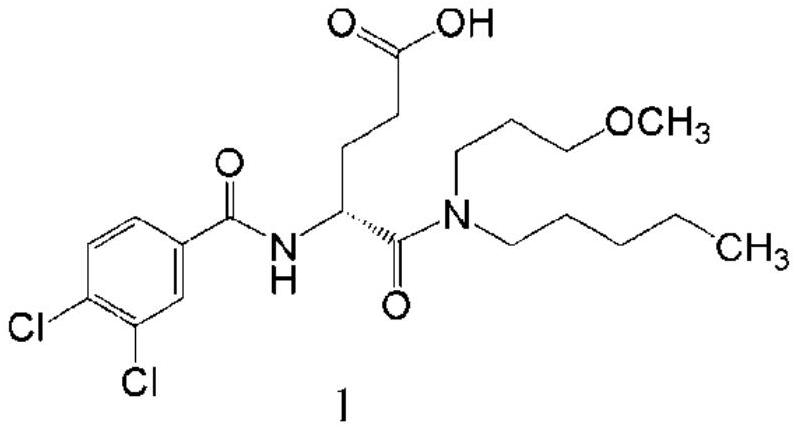
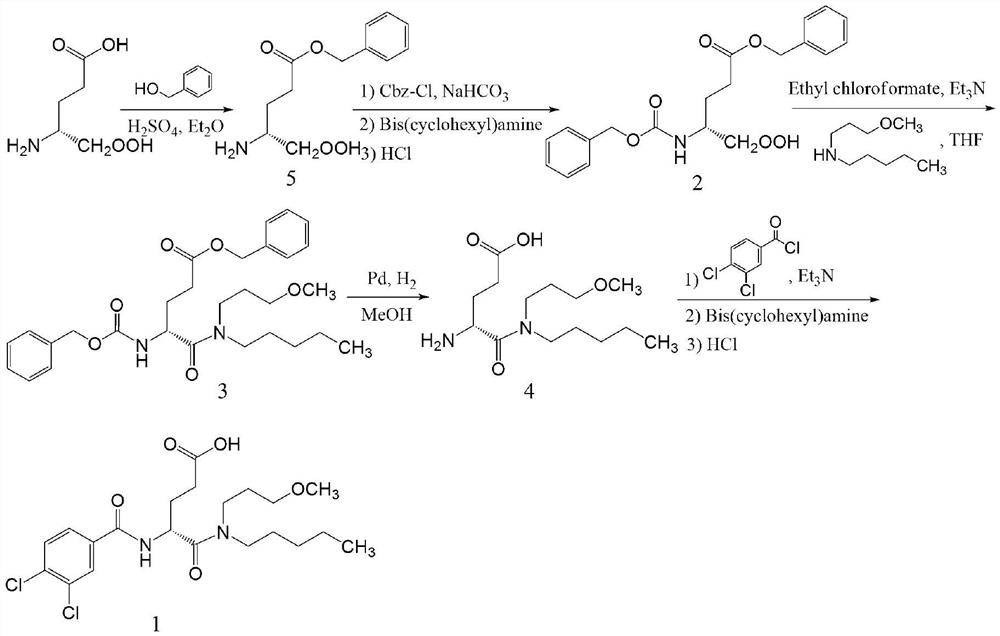
A kind of preparation method of dexloglutamine
A technology of dexloglutamine and glutamic acid, which is applied in the field of medicine, can solve the problems of cumbersome post-processing and low total yield, and achieve the effect of simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1. Preparation of N-Boc-D-glutamic acid-5-benzyl ester (compound 6)
[0042] Add 4.84g (0.02mol, 98%) of D-glutamic acid 5-benzyl ester (compound 5), 60mL of tetrahydrofuran and 20mL of N,N-dimethylformamide mixed solution in a 250mL flask, 8.1g of Triethylamine and 6.55 g of di-tert-butyl dicarbonate. After heating to 60°C for 4 hours, evaporate tetrahydrofuran and N,N-dimethylformamide to dryness under reduced pressure, and dissolve the obtained oil in 100 mL of 0.25 M KHSO 4 The solution was acidified to pH 2.0-3.0 and stirred for 15 min. Extract with ethyl acetate (30 mL) 4 times. After drying, the solvent was evaporated under reduced pressure, and the obtained oily liquid was directly used in the next reaction with a yield of 91%. IR(KBr):3421,2977,2930,1718,1508,1457,1393,1368,1251,1165,1054,750,698cm -1 ; 1 H NMR (400MHz, CDCl 3 )δ7.23(d,J=4.6Hz,5H),5.00(s,2H),4.19(s,1H),4.12(s,1H),2.38(d,J=7.0Hz,3H),2.16- 2.09(m,3H),1.32(s,10H). 13 C NMR (101MHz,...
Embodiment 2
[0043] Example 2. Preparation of Compound 7
[0044] Add 6.9g of N-Boc-D-glutamic acid-5-benzyl ester (compound 6) into a 100mL bottle, dissolve it in 50mL of tetrahydrofuran, stir and cool to -10°C, add 2.02g of N-methylmethanol 2.73 g of isobutyl chloroformate was added dropwise while vigorously stirring (completely added within 10 min). After stirring the reaction at -10°C for 20 minutes, add 3.25 g of N-(3-methoxypropyl)-pentylamine in three batches The temperature was reacted for 5h. The filtrate was evaporated to dryness under reduced pressure, and the obtained residue was added to 60 mL of ethyl acetate, and extracted successively with 0.5M hydrochloric acid (2×30 mL), 1M sodium bicarbonate (2×20 mL) and saturated NaCl (2×20 mL). Anhydrous Na 2 SO 4 After drying, the solvent was evaporated to dryness, and 50 mL of 2.4 M HCl ethyl acetate solution was added to the obtained viscous light yellow liquid, and reacted at room temperature for 1 h. After the reaction, 20 ...
Embodiment 3
[0045] Example 3. Preparation of compound 8
[0046] 7.56g of compound 7 was placed in a 250mL flask, and 40mL of 0.5M Na 2 CO 3 and 20 mL of tetrahydrofuran. After dissolving, 20 mL of THF solution containing 3.89 g of 3,4-dichlorobenzoyl chloride was added dropwise at 0-5°C (dropped over 30 min), and the reaction was continued at this temperature for 1 h. After the reaction, tetrahydrofuran was evaporated to dryness, and the obtained aqueous phase was diluted with 60 mL of dichloromethane, filtered to remove insoluble impurities, and the filtrate was separated into two phases. The organic phase was sequentially washed with 20 mL of 1M NaHCO 3 , 1M hydrochloric acid and saturated NaCl aqueous solution were extracted twice respectively, and the solvent was evaporated to dryness to obtain 9.71 g of light yellow oily liquid (ie compound 8), with a yield of 89%. IR(KBr):3422,2960,2931,2873,1735,1711,1642,1610,1497,1455,1387,1366,1250,1172,1118,1050,778,750,698cm -1 ; 1 H NM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com