Application of heteroatom-containing triazine covalent organic framework material in photocatalysis
A covalent organic framework and heteroatom technology, applied in organic compound/hydride/coordination complex catalysts, inorganic chemistry, physical/chemical process catalysts, etc., can solve the problem of low photocatalytic efficiency and achieve high catalytic efficiency , mild application, rich composition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Add 0.06g of 3,6-dialdehyde ethylcarbazole, 0.12g of terephthalmidine, and 0.3g of cesium carbonate into 10mL of DMSO, and react at 120°C for 120h. After the reaction is completed, filter with suction, then wash with 150mL dilute hydrochloric acid, repeat three times, then wash with 150mL acetone, repeat three times, and finally wash with 150mL N,N dimethylformamide, and repeat washing three times, washing to remove residual Oligomers and catalysts. The obtained solid was subjected to Soxhlet extraction with tetrahydrofuran for 24 h, and vacuum-dried to obtain the product with a yield of 70%.
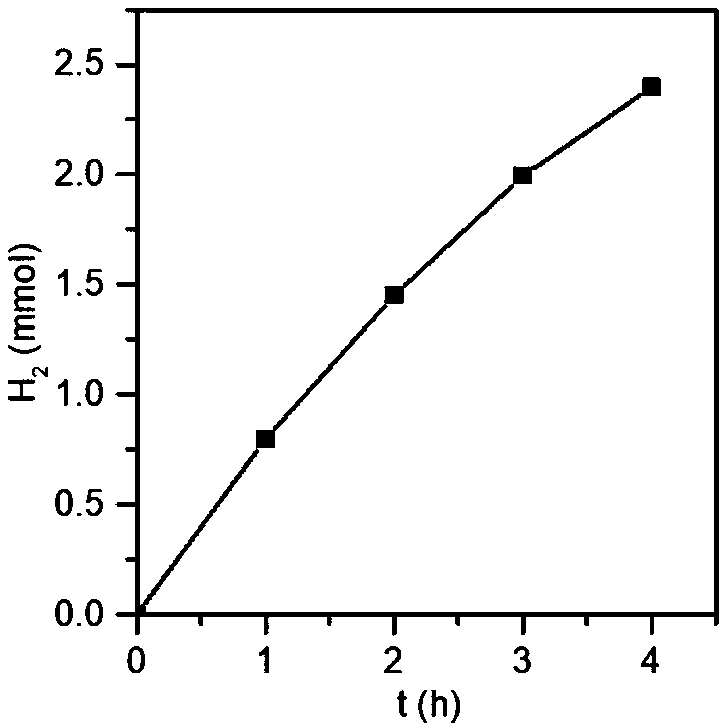
[0037] Get 4 mg of the product of Example 1, 80 mL of an aqueous solution of Rhodamine B at 20 ppm, first dark-treat for 1 h, and then test at a wavelength of visible light (λ≥420 nm). figure 1 It is the change of the ultraviolet response of methyl orange with the photodegradation time when the triazine ring covalent organic framework material obtained in Example 1 is used as a ...
Embodiment 2
[0040] Add 0.08g of 4,8-diformyl-benzothiadiazole, 0.12g of phthalimidine, and 0.3g of cesium carbonate into 10mL of DMF, and react at 100°C for 120h. After the reaction is completed, filter with suction, then wash with 150mL dilute hydrochloric acid, repeat three times, then wash with 150mL acetone, repeat three times, and finally wash with 150mL N,N dimethylformamide, and repeat washing three times, washing to remove residual Oligomers and catalysts. The obtained solid was subjected to Soxhlet extraction with tetrahydrofuran for 24 h, and vacuum-dried to obtain the product with a yield of 85%.
[0041] Take 4 mg of the product of Example 2, 80 mL of an aqueous solution of 20 ppm methyl orange, first dark-treat for 1 h, and then test under ultraviolet and visible light irradiation (λ≥350 nm). image 3 It is the change of the ultraviolet response of methyl orange with the photodegradation time when the triazine ring covalent organic framework material obtained in Example 2 is...
Embodiment 3
[0044] Add 0.08g of 2,6-diformylpyridine, 0.12g of phthalimidine, and 0.3g of cesium carbonate into 10mL of DMSO, and react at 150°C for 120h. After the reaction is completed, filter with suction, then wash with 150mL dilute hydrochloric acid, repeat three times, then wash with 150mL acetone, repeat three times, and finally wash with 150mL N,N dimethylformamide, and repeat washing three times, washing to remove residual Oligomers and catalysts. The obtained solid was subjected to Soxhlet extraction with tetrahydrofuran for 24 h, and vacuum-dried to obtain the product with a yield of 64%.
[0045] Take 4 mg of the product of Example 3, 80 mL of an aqueous solution of 20 ppm methylene blue, first dark-treat for 1 h, and then test at a visible light wavelength (λ≥420 nm). Figure 5 It is the change of the ultraviolet response of methyl orange with the photodegradation time when the triazine ring covalent organic framework material obtained in Example 3 is used as a photocatalyst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com