Anthraquinonotriazole antibiotic nucleoside analog, synthetic method and application in preparation of antineoplastic or antiviral drug
A technology for anthraquinone triazole and nucleoside analogs, which is used in the fields of chemistry and medicine to achieve good anti-tumor and anti-viral activities, and to overcome the effects of toxic and side effects such as cardiotoxicity and drug resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0043]
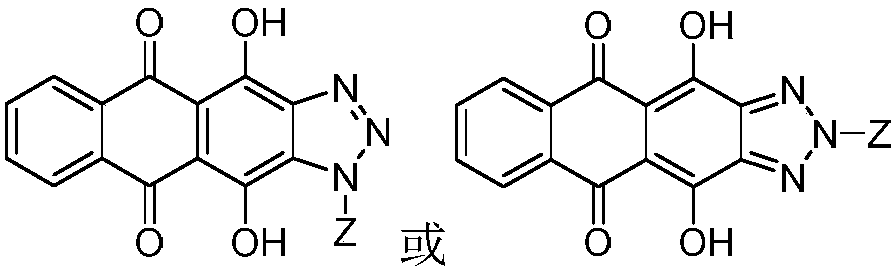
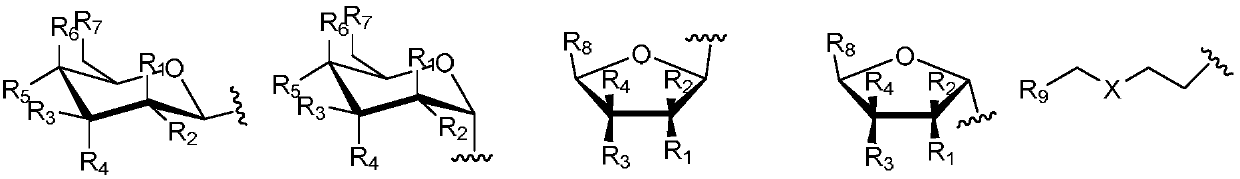
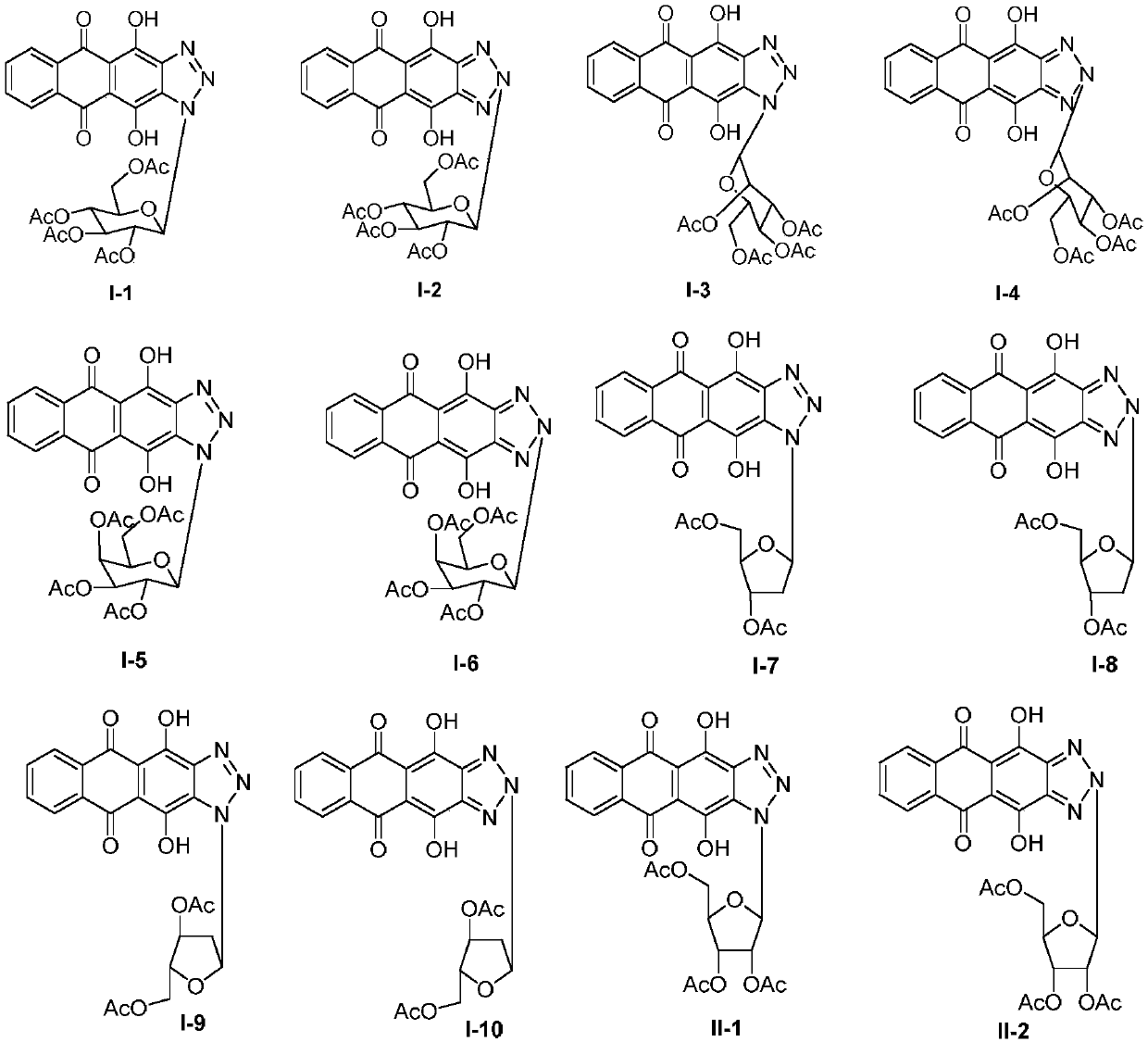
[0044] 1. Synthesis of glycosyl ligand 4,11-dihydroxyanthra[2,3-d][1,2,3]triazole-5,10-dione
[0045] Step S1: Add 6 g of compound 1 to 90.0 mL of glacial acetic acid, then add 5.0 mL of concentrated nitric acid, heat and stir at 60°C for 3.5 h, filter the reaction solution, wash the filter cake with AcOH and water, and then dry to obtain compound 2 , the yield is 62%;
[0046] Step S2: Dissolve 500 mg of hydroxylamine hydrochloride in 15.0 mL of absolute ethanol, add 300 mg of potassium hydroxide, stir for 30 min, filter out KCl white solid to obtain the filtrate, add 500 mg of compound 2 to the above filtrate, stir for 1.5 h, filter, filter The cake was washed with absolute ethanol and water respectively, and then dried in an oven at 30°C to obtain compound 3 with a yield of 96%;
[0047] Step S3: Add 20.6g of sodium sulfide to 120.0mL of a mixed solvent of ethanol and water with a volume ratio of 3:1, then add 2.6g of compound 3 to the above solution, then add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com