Amide aromatic phenol antibacterial peptide analogue with antibacterial activity and preparation method thereof
A naphthol-like antibacterial peptide and antibacterial activity technology, applied in the field of amide aromatic antibacterial peptide mimetics and preparation thereof, can solve the problems of high production cost, easy degradation and weak selectivity of natural antibacterial peptides, and achieve the solution of drug-resistant bacteria. problem, excellent antibacterial activity, highly selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
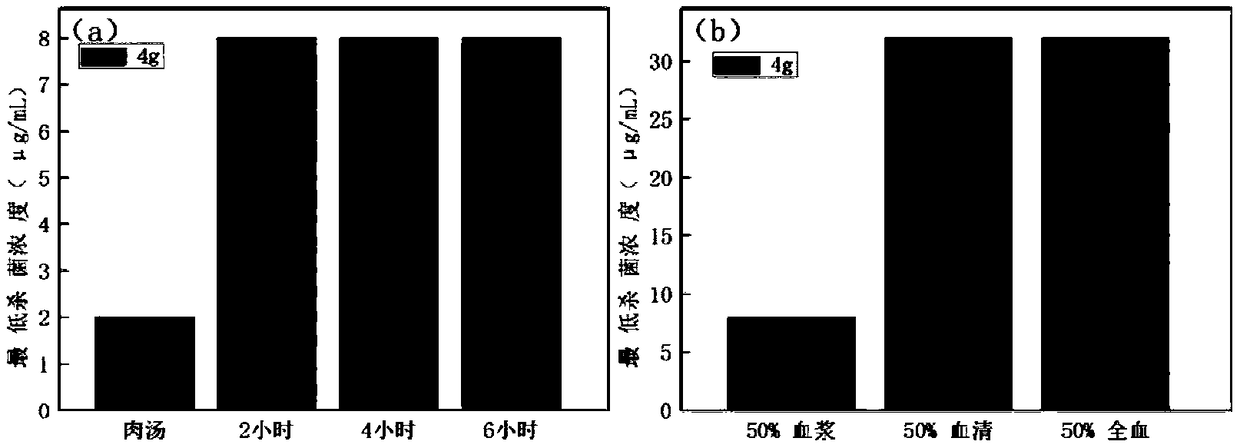
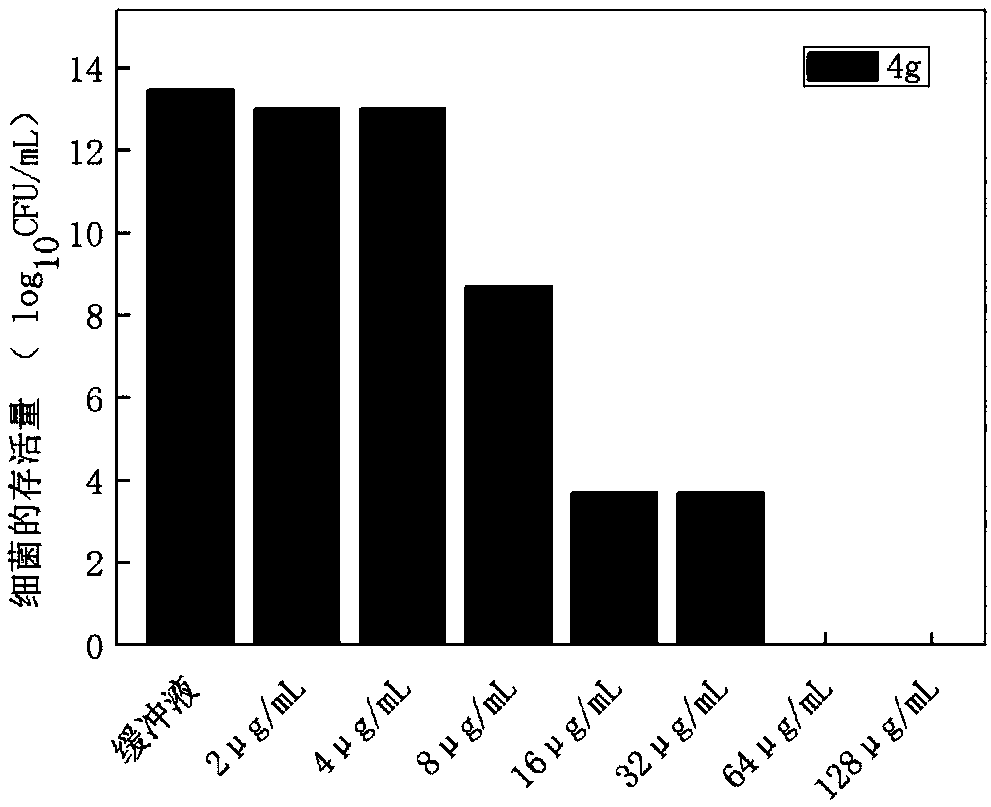
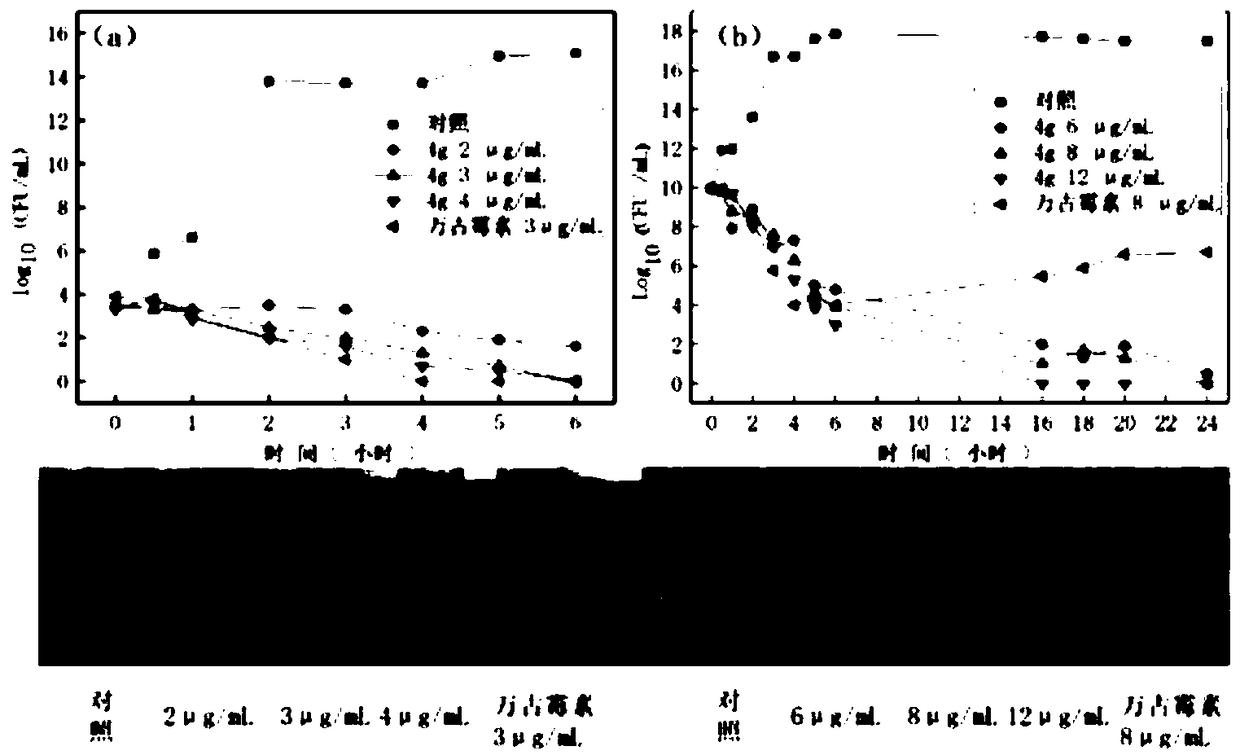
Image
Examples
Embodiment 1
[0073] The preparation of embodiment 1 compound 1a
[0074] In a round bottom flask (500 mL), weigh 1,4-bis(2-hydroxyethoxy)benzene (5 g, 25 mmol) and triphenylphosphine (15.7 g, 60 mmol) and dissolve in anhydrous acetonitrile (120 mL) , and then keeping the temperature at 0° C., carbon tetrabromide (19.9 g, 60 mmol) was slowly added to the above system. Then the temperature was raised to room temperature 25° C., and stirred for 4 hours under the protection of nitrogen. After the reaction, add ice water (200mL) to the system to precipitate the product, and the solid was obtained by filtration and washed with methanol / water (3:2, 3×100mL) for 3-4 times. The crude product was further purified by recrystallization in methanol to obtain pure product.
Embodiment 2
[0075] 1a: 1 H NMR (400MHz, CDCl 3 )δ6.86(s,4H),4.24(t,J=6.3Hz,4H),3.61(t,J=6.3Hz,4H). 13 C NMR (101MHz, CDCl 3 ) δ152.85, 116.12, 68.74, 29.25. Preparation of Example 2 Compound 1b
[0076] In a round bottom flask (250mL), take hydroquinone (8g, 72.65mmol, 1eq) and dissolve it in acetone (150mL), then add dibromopropane (44g, 217.96mmol, 3eq) and potassium carbonate (45.18g, 326.94mmol, 4.5eq) stirred to dissolve the compound, added magneton, and heated to reflux for 24 hours under the protection of nitrogen. After the reaction was completed, the system was lowered to room temperature, filtered, and the filter residue was washed 3-4 times with dichloromethane. The filtrate was washed 3-4 times with water, then washed 3-4 times with saturated aqueous sodium chloride solution, and finally the solution was dried with anhydrous sodium sulfate, filtered, evaporated to dryness with a rotary evaporator, and separated by a silica gel column (petroleum ether: ethyl acetate Ester ...
Embodiment 3
[0078] Preparation of Compound 1c in Example 3: The dibromoalkane used was dibromobutane, which was separated on a silica gel column (petroleum ether: ethyl acetate = 30:1). The preparation method was the same as in Example 2.
[0079] 1c: 1 H NMR (400MHz, CDCl 3 )δ6.81(s,4H),3.93(t,J=6.1Hz,4H),3.48(t,J=6.7Hz,4H),2.11–2.00(m,4H),1.96–1.83(m,4H ). 13 C NMR (101MHz, CDCl 3 )δ153.11, 115.44, 77.41, 77.09, 76.77, 67.49, 33.55, 29.54, 28.03.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com