Magnesium oxide composite material for fluorine removal of water body and preparation method and application thereof
A composite material, magnesium oxyfluoride technology, applied in chemical instruments and methods, alkali metal compounds, alkali metal oxides/hydroxides, etc., can solve problems such as difficulty in application and loss of active components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A magnesium oxide composite material for removing fluoride from water, which is composed of the following raw materials in weight fractions: 30 parts of magnesium oxide, 2 parts of adhesive, and 40 parts of carrier particles; wherein the adhesive is cellulose acetate; the carrier particles are Activated carbon.
[0023] Preparation:
[0024] (1) Dissolve the adhesive with a solvent; the ratio of adhesive to solvent is 1:5 (w:v);
[0025] (2) Add the carrier particles into the adhesive solution, stir and mix evenly;
[0026] (3) Add magnesium oxide and mix well. Make magnesium oxide-adhesive agent-carrier particle composite;
[0027] (4) Place the magnesium oxide-adhesive agent-carrier particle composite in an oven for high-temperature curing at a temperature of 180° C. and a high-temperature curing time of 30 minutes to obtain a magnesium oxide composite material; the solvent is acetone.
[0028] [Effect of removing fluorine in Example 1]
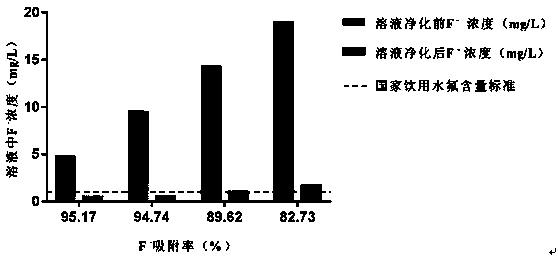
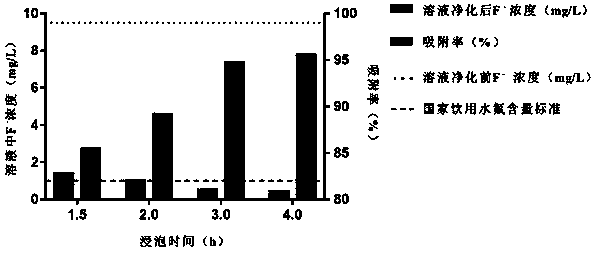
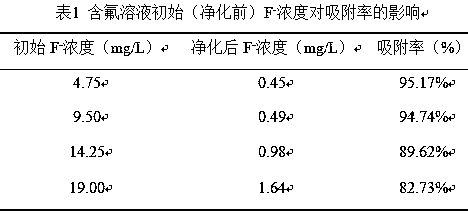
[0029] Fluoride removal ...
Embodiment 2
[0034] A water body defluoridation magnesium oxide composite material, which is composed of the following raw materials in weight fractions: 50 parts of magnesium oxide, 10 parts of adhesive, 60 parts of carrier particles; wherein the adhesive is polystyrene; the carrier particles are Chitosan.
[0035] Preparation:
[0036] (1) Dissolve the adhesive with a solvent; the ratio of adhesive to solvent is 1:30 (w:v);
[0037] (2) Add the carrier particles into the adhesive solution, stir and mix evenly;
[0038] (3) Add magnesium oxide and mix well. Make magnesium oxide-adhesive agent-carrier particle composite;
[0039] (4) Place the magnesium oxide-adhesive agent-carrier particle composite in an oven for high-temperature curing, the temperature is 150°C, and the high-temperature curing time is 20 minutes to obtain a magnesium oxide composite material; the solvent is dimethylformamide:tetrahydrofuran =4:6(v:v).
[0040] [Effect of removing fluorine in Example 2]
[0041] In...
Embodiment 3
[0046] A magnesium oxide composite material for removing fluoride from water, which is composed of the following raw materials in weight fractions: 45 parts of magnesium oxide, 18 parts of adhesive, and 55 parts of carrier particles; wherein the adhesive is cellulose acetate; the carrier particles are diatomite.
[0047] Preparation:
[0048] (1) Dissolve the adhesive with a solvent; the ratio of adhesive to solvent is 1:25 (w:v);
[0049](2) Add the carrier particles into the adhesive solution, stir and mix evenly;
[0050] (3) Add magnesium oxide and mix well. Make magnesium oxide-adhesive agent-carrier particle composite;
[0051] (4) Place the magnesium oxide-adhesive agent-carrier particle composite in an oven for high-temperature curing at a temperature of 230° C. and a high-temperature curing time of 150 minutes to obtain a magnesium oxide composite material; the solvent is acetone.
[0052] [Example 3 defluoridation effect]
[0053] The curing effect of magnesium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com