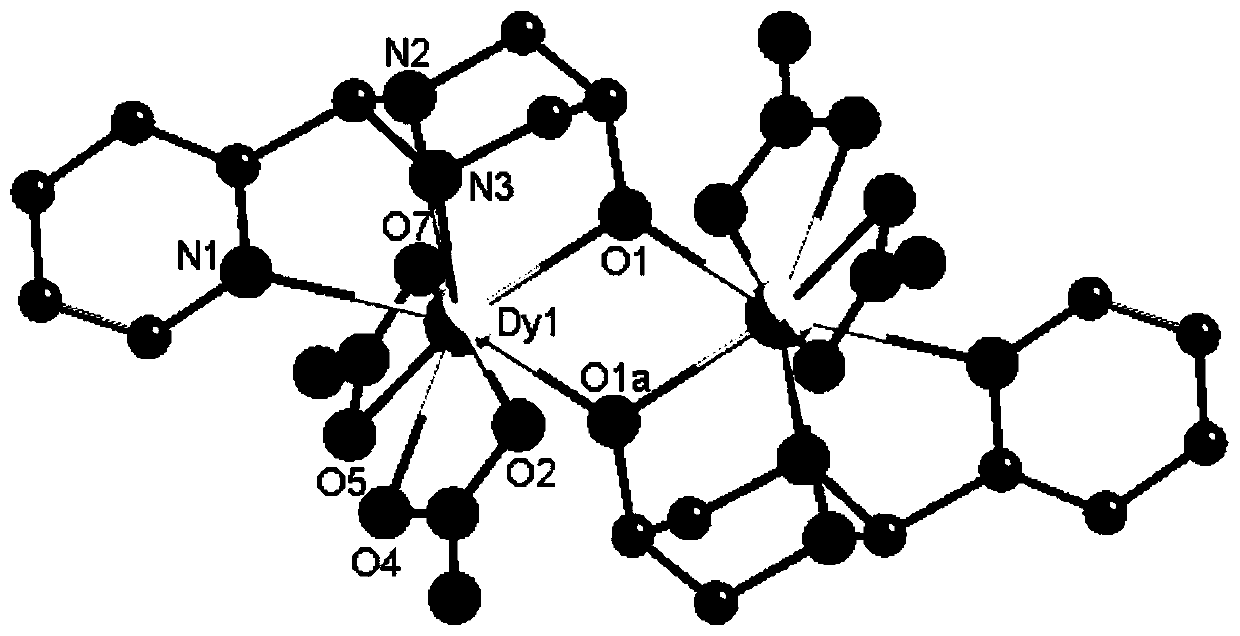
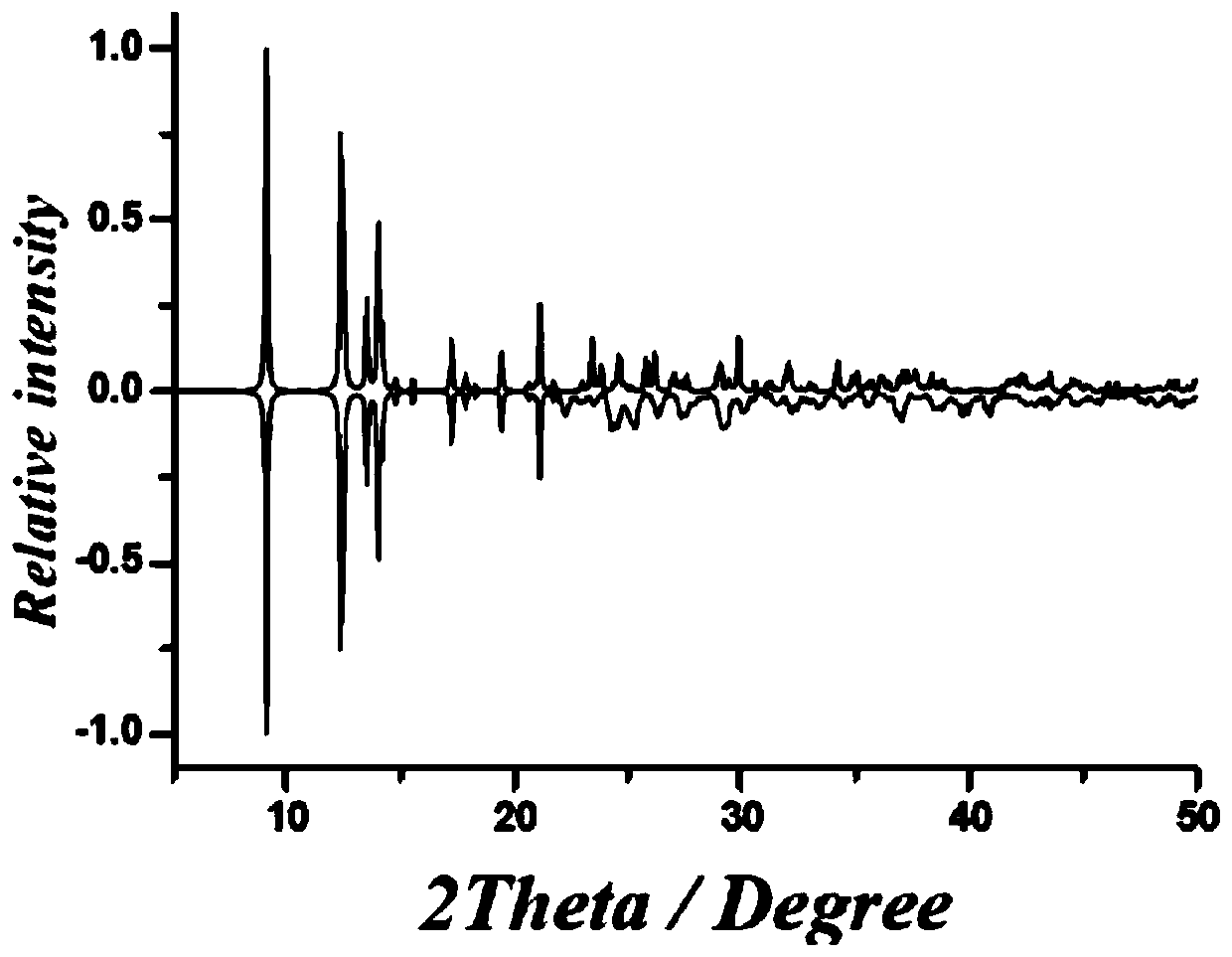
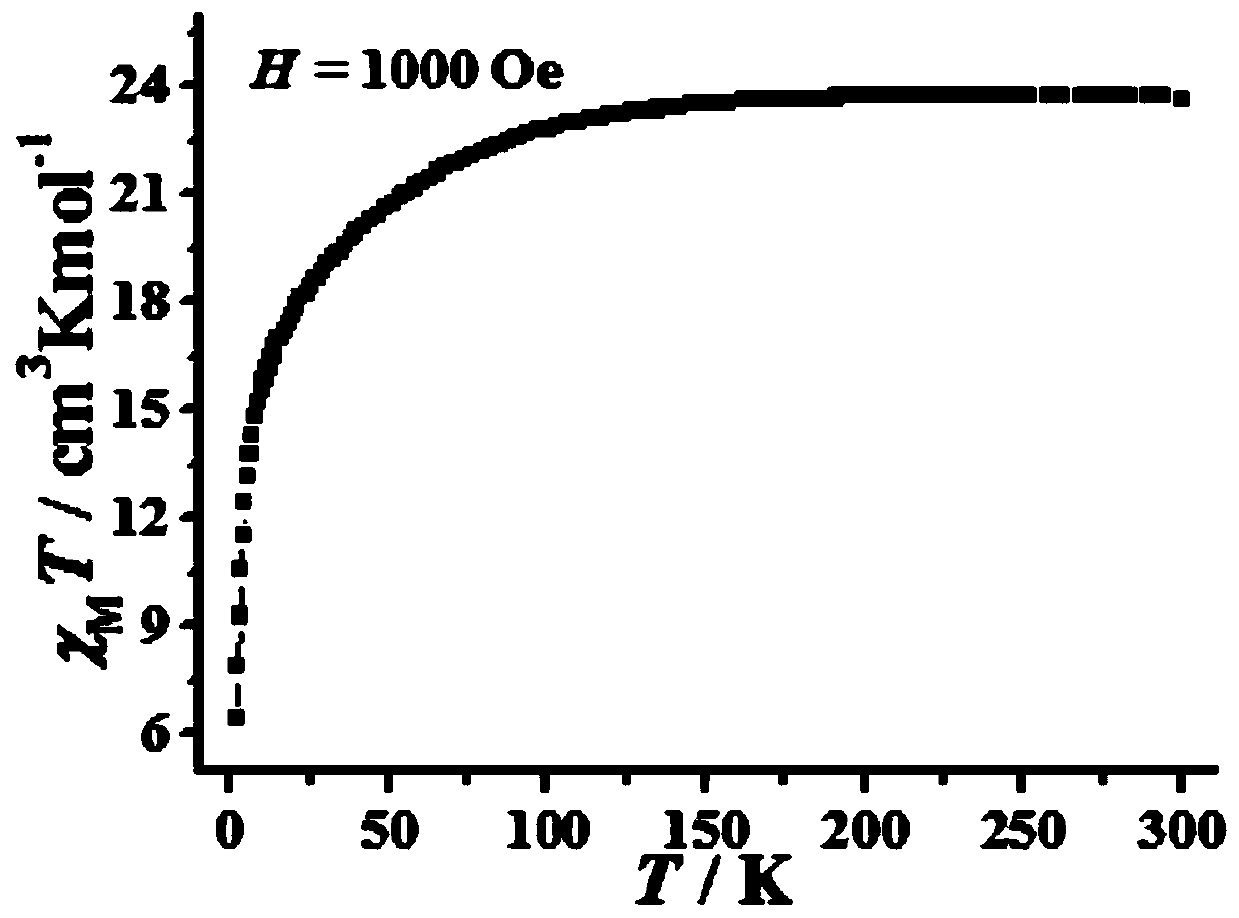
2-Pyridineformaldehyde acetal 1,3 diamino-2-propanol dinuclear dysprosium cluster compound and its synthesis method and application
A technology of pyridine formaldehyde and synthesis method, which is applied to the 3/13 group organic compounds without C-metal bonds, compounds containing group 3/13 elements of the periodic table, organic chemical methods, etc., which can solve the inconvenience of modeling calculations, etc. problem, to achieve the effect of easy operation and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1) Weigh 0.1071g (1mmol) of 2-pyridinecarbaldehyde and dissolve it in 5mL of acetonitrile, and dissolve 0.0451g (0.5 mmol) of 1,3-diamino-2-propanol in 5mL of ethanol. The acetonitrile solution of pyridine formaldehyde is slowly added to the ethanol solution of 1,3-diamino-2-propanol, and mixed evenly to obtain the aldehyde-amine mixture, which is set aside;
[0028] 2) Weigh the metal salt Dy(NO 3 ) 3 ·6H 2 Add O (0.1mmol, 45.6mg) into a glass bottle with a volume of about 20 mL, pipette 1 mL of the above-mentioned aldehyde-amine mixture into the glass bottle, and then add 1.5 mL of acetonitrile to make the ethanol in the glass bottle The volume ratio with acetonitrile is 1:4 (0.5mL and 2mL respectively), then add 6 μL triethylamine to it, shake well (the pH of the solution at this time=7.5); then seal the glass bottle mouth with aluminum foil , put the bottle cap on, put the glass bottle in an oven at 60°C for 24 hours, take it out, wrap it with cotton wool and put...
Embodiment 2
[0065] Repeat Example 1, the difference is:
[0066] 1) After adding 1mL of aldehyde-amine mixture into the glass bottle, add 1mL of acetonitrile to it, so that the volume ratio of ethanol and acetonitrile in the mixed solvent is 1:3;
[0067] 2) adjust the pH of the system to 7.2 with triethylamine;
[0068] 3) The reaction is carried out at 50° C., and the reaction time is 48 hours.
[0069] Colorless long strip crystals were obtained with a yield of 22.1%. The resulting product was characterized for its structure, and it was determined that the product was the target product [Dy 2(C 9 h 12 N 3 O) 2 (NO 3 ) 4 ]. Characterization of the magnetic properties of the obtained product shows that the obtained product has a single-molecule magnet behavior.
Embodiment 3
[0071] Repeat Example 1, the difference is:
[0072] 1) After adding 1mL of aldehyde-amine mixture to the glass bottle, add 2mL of acetonitrile to it, so that the volume ratio of ethanol and acetonitrile in the mixed solvent is 1:5;
[0073] 2) adjust the pH of the system to 7.6 with triethylamine;
[0074] 3) The reaction is carried out at 80° C., and the reaction time is 30 h.
[0075] Colorless long crystals were obtained with a yield of 21.5%. The resulting product was characterized for its structure, and it was determined that the product was the target product [Dy 2 (C 9 h 12 N 3 O) 2 (NO 3 ) 4 ]. Characterization of the magnetic properties of the obtained product shows that the obtained product has a single-molecule magnet behavior.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com