Solid-phase synthesis method of beta-amyloid peptide 1-42
A solid-phase synthesis method and technology of amyloid peptides, which are applied in the field of peptide solid-phase synthesis, can solve the problems of default peptide impurities, reduce the sensitivity of ninhydrin, and increase the difficulty of amino acid coupling.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
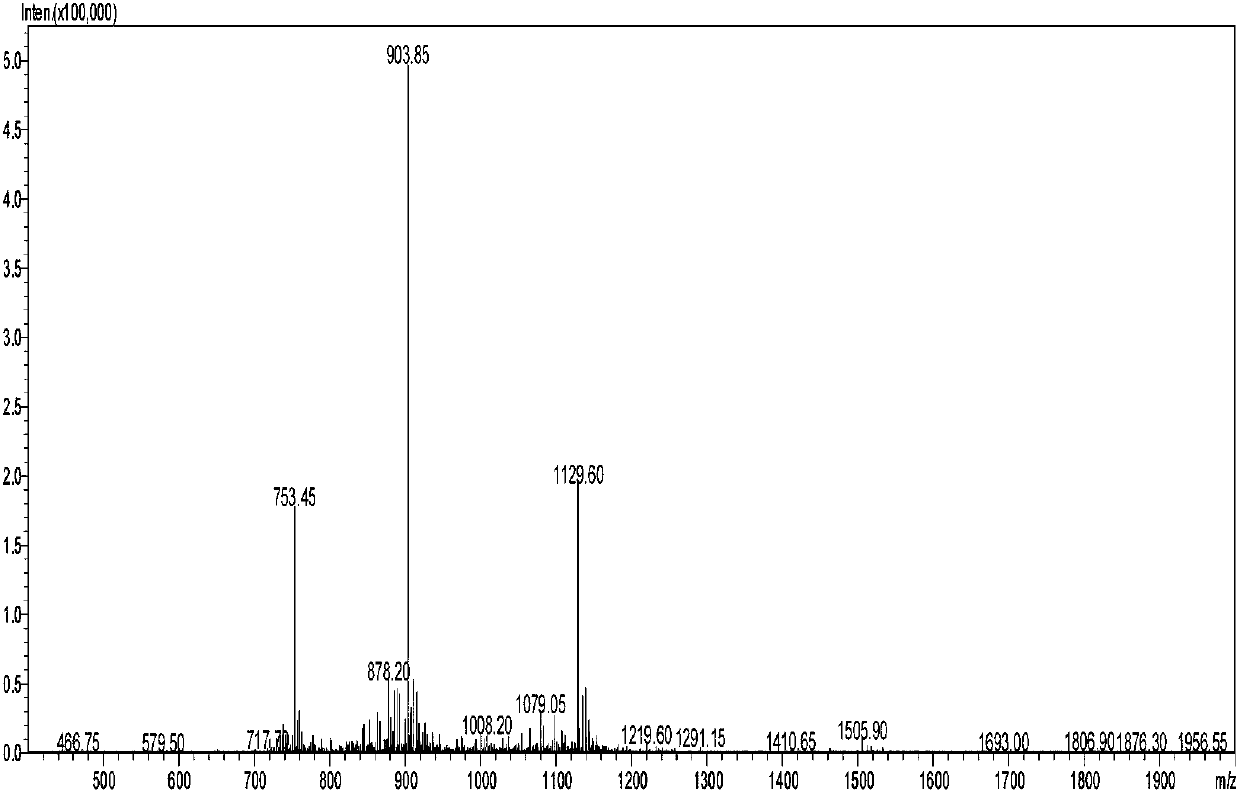
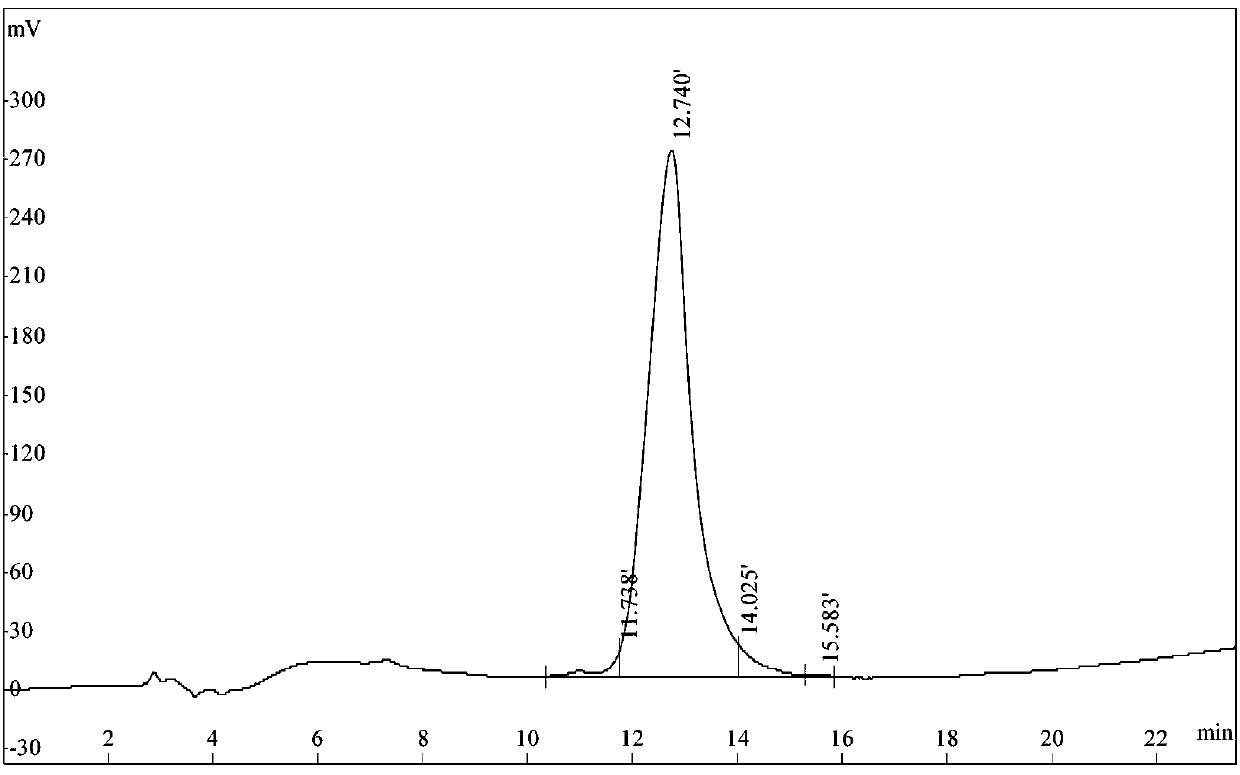
Image
Examples
Embodiment 1
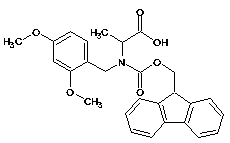
[0074] In this example only Ala 21 Select Fmoc-(Fmoc-Hmb)-Ala-OH as the coupling amino acid, Gly 25 , Gly 29 , Gly 33 , Gly 38 Select Fmoc-(Fmoc-Hmb)-Gly-OH as the coupling amino acid;
[0075] (1) Preparation of Fmoc-Ala-HMP-TentaGel resin
[0076] Weigh 20 grams of HMP-TentaGel resin (substitution degree 1.0mmol / g) into a glass reaction column, add DCM to soak for 30 minutes, drain, add Fmoc-Ala-OH 3.74g (12mmol), TBTU 3.86g (12mmol) , HOBT 1.63g (12mmol) DIEA 3.1g (24mmol), add the DMF / NMP mixture solution with a volume ratio of 1:1 to dissolve, react with nitrogen gas bubbling for 4 hours, drain, wash with DMF for 3 times, and then add the volume ratio Acetic anhydride of 2:2:1: pyridine: the mixed solution of DMF, react 1 hour, drain, wash 6 times with DMF, drain, obtain Fmoc-Ala-HMP-TentaGel resin 22.3g (10mmol Loading=0.45mmol / g).
[0077] (2) Preparation of β-amyloid peptide 1-42 resin peptide
[0078] Add 20% volume ratio PIP / DMF decapping reagent to Fmoc-Ala...
Embodiment 2
[0083] In this example only Ala 21 , Ala 30 Use Fmoc-(Fmoc-Hmb)-Ala-OH as the coupling amino acid, Gly 25 , Gly 37 Select Fmoc-(Fmoc-Hmb)-Gly-OH as the coupling amino acid;
[0084] (1) Preparation of Fmoc-Ala-HMP-TentaGel resin
[0085] Weigh 20 grams of HMP-TentaGel resin (substitution degree 1.0mmol / g) into a glass reaction column, add DCM to soak for 30 minutes, drain, add Fmoc-Ala-OH 3.74g (12mmol), TBTU 3.86g (12mmol) , HOBT 1.63g (12mmol) DIEA 3.1g (24mmol), add the DMF / NMP mixture solution with a volume ratio of 1:1 to dissolve, react by bubbling nitrogen for 6 hours, drain, wash with DMF for 3 times, and then add the volume ratio Acetic anhydride of 2:2:1: pyridine: the mixed solution of DMF, react 1.5 hours, drain, wash 6 times with DMF, drain, obtain Fmoc-Ala-HMP-TentaGel resin 22.3g (10mmol Loading=0.45mmol / g).
[0086] (2) Preparation of β-amyloid peptide 1-42 resin peptide
[0087]Add 20% volume ratio PIP / DMF decapping reagent to Fmoc-Ala-HMP-TentaGel res...
Embodiment 3
[0092] In this example only Ala 21 , Ala 30 Use Fmoc-Dmb-Ala-OH as coupling amino acid, Gly 25 , Gly 37 Select Fmoc-Dmb-Gly-OH as the coupling amino acid;
[0093] (1) Preparation of Fmoc-Ala-HMP-TentaGel resin
[0094] Weigh 20 grams of HMP-TentaGel resin (substitution degree 1.0mmol / g) into a glass reaction column, add DCM to soak for 30 minutes, drain, add Fmoc-Ala-OH 3.74g (12mmol), TBTU 3.86g (12mmol) , HOBT 1.63g (12mmol) DIEA 3.1g (24mmol), add the DMF / NMP mixture solution with a volume ratio of 1:1 to dissolve, react by bubbling nitrogen for 8 hours, drain, wash with DMF for 3 times, and then add the volume ratio 2:2:1 acetic anhydride:pyridine:DMF mixed solution, reacted for 0.5 hours, drained, washed 6 times with DMF, drained to obtain Fmoc-Ala-HMP-TentaGel resin 22.3g (10mmol Loading=0.45mmol / g).
[0095] (2) Preparation of β-amyloid peptide 1-42 resin peptide
[0096] Add 20% volume ratio PIP / DMF decapping reagent to Fmoc-Ala-HMP-TentaGel resin 22.3g (10mmo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com