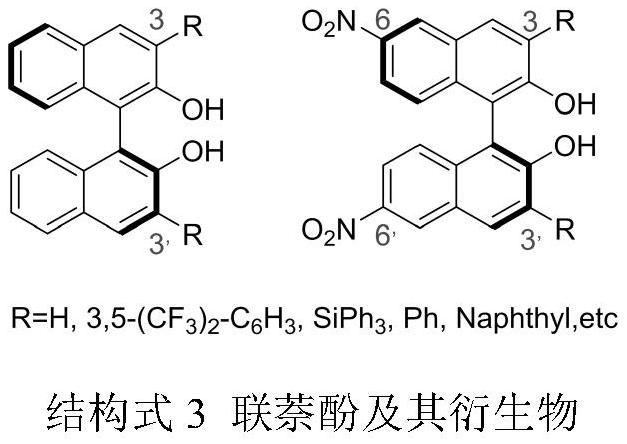
A kind of synthetic method of novel chiral nitrobinaphthol and its derivatives
A technology of nitrobinaphthol and synthesis method, which is applied in the field of organic synthesis, can solve the problems of many by-products, pollute the environment, and low atom economy, and achieve the effect of less reaction by-products and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the target product in the present embodiment is:
[0029] Specifically, it is realized by the following steps:
[0030] Add 1mmol (R)-1,1'-binaphthol into the reaction flask, dissolve it in 5mL tetrahydrofuran, add 422uL tert-butyl nitrite, stir at room temperature, and react for 12 hours. After the reaction, use rotary evaporation The organic solvent was removed, and the product nitrobinaphthol was obtained by column chromatography, and the reaction yield was 92%.
Embodiment 2
[0031] Embodiment 2: the target product in the present embodiment is:
[0032] Specifically, it is realized by the following steps:
[0033] Add 1mmol (R)-1,1'-binaphthol into the reaction flask, dissolve it in 5mL tetrahydrofuran, add 422uL tert-butyl nitrite, stir at room temperature, and react for 12 hours. After the reaction, use rotary evaporation The organic solvent was removed by using an instrument, and the product nitrobinaphthol was obtained by column chromatography, and the reaction yield was 95%.
Embodiment 3
[0034] Embodiment 3: the target product in the present embodiment is:
[0035] Specifically, it is realized by the following steps:
[0036] Add 1mmol (R)-1,1'-binaphthol into the reaction flask, dissolve it in 2mL tetrahydrofuran, add 422uL tert-butyl nitrite, stir at room temperature, and react for 12 hours. After the reaction, use rotary evaporation The organic solvent was removed, and the product nitrobinaphthol was obtained by column chromatography, and the reaction yield was 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com