Method for preparing thionocarbamates and co-producing 2-mercaptoethanol or O-alkylthioethyl xanthogenate
A technology of alkylthioethyl yellow and alkylthioethyl, which is applied in mercaptan preparation, flotation, solid separation, etc., and can solve the problem of benzylmercaptan's unpleasant smell, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Embodiment 1: Preparation of O-isopropyl-S-hydroxyethyl xanthate
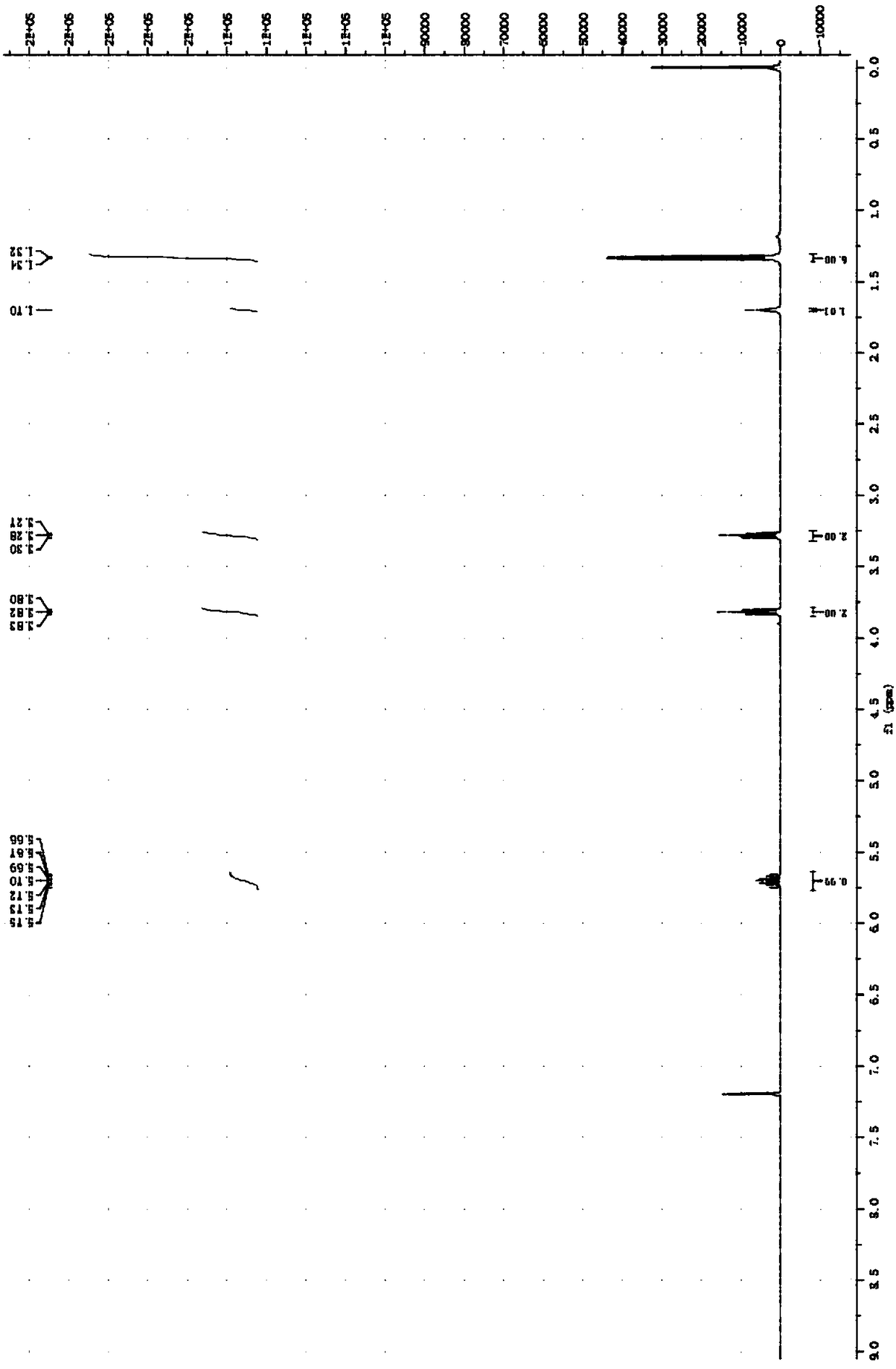
[0094] Add 8.13 parts of 2-chloroethanol with a purity of 99% into the reactor, add 18.9 parts of sodium isopropylxanthate with a purity of 83.54% in batches, stir while adding, then add 15 parts of distilled water, and heat up to the bottle The temperature is 60°C, after 5 hours of constant temperature reaction, cool to room temperature, and separate the liquid to obtain the oil phase O-isopropyl-S-hydroxyethyl xanthate. The analysis shows that O-isopropyl-S-hydroxyethyl xanthate The yield of acid ester was 88.3%. The product was characterized after being purified by column chromatography, the O-isopropyl-S-hydroxyethyl xanthate 1 H NMR as figure 1 As shown, the infrared spectrum is shown in Image 6 shown.
Embodiment 2
[0095] Embodiment 2: the preparation of O-isobutyl-S-hydroxyethyl xanthate
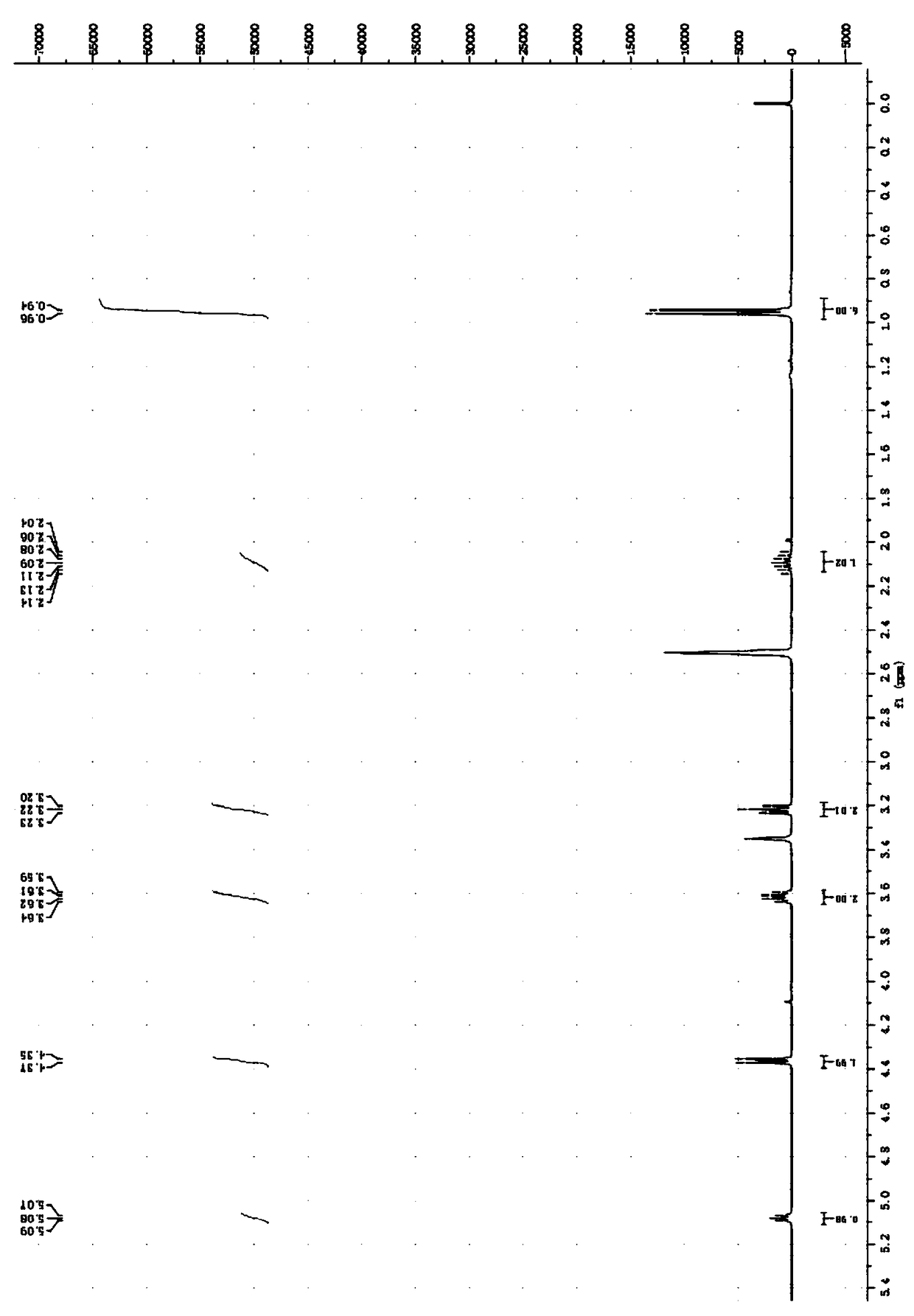
[0096] Add 8.13 parts of 2-chloroethanol with a purity of 99% into the reactor, add 19.32 parts of sodium isobutyl xanthate with a purity of 89.0% in batches, stir while adding, then add 15 parts of distilled water, and heat up to the bottle The temperature is 50°C, after 7 hours of constant temperature reaction, cool to room temperature, and separate the liquid to obtain the oil phase O-isobutyl-S-hydroxyethyl xanthate. The analysis shows that O-isobutyl-S-hydroxyethyl xanthate The yield of acid ester was 78.2%. The product was characterized after being purified by column chromatography, the O-isobutyl-S-hydroxyethyl xanthate 1 H NMR as figure 2 As shown, the infrared spectrum is shown in Figure 7 shown.
Embodiment 3
[0097] Embodiment 3: the preparation of O-isopropyl-N-ethyl thiocarbamate and 2-mercaptoethanol
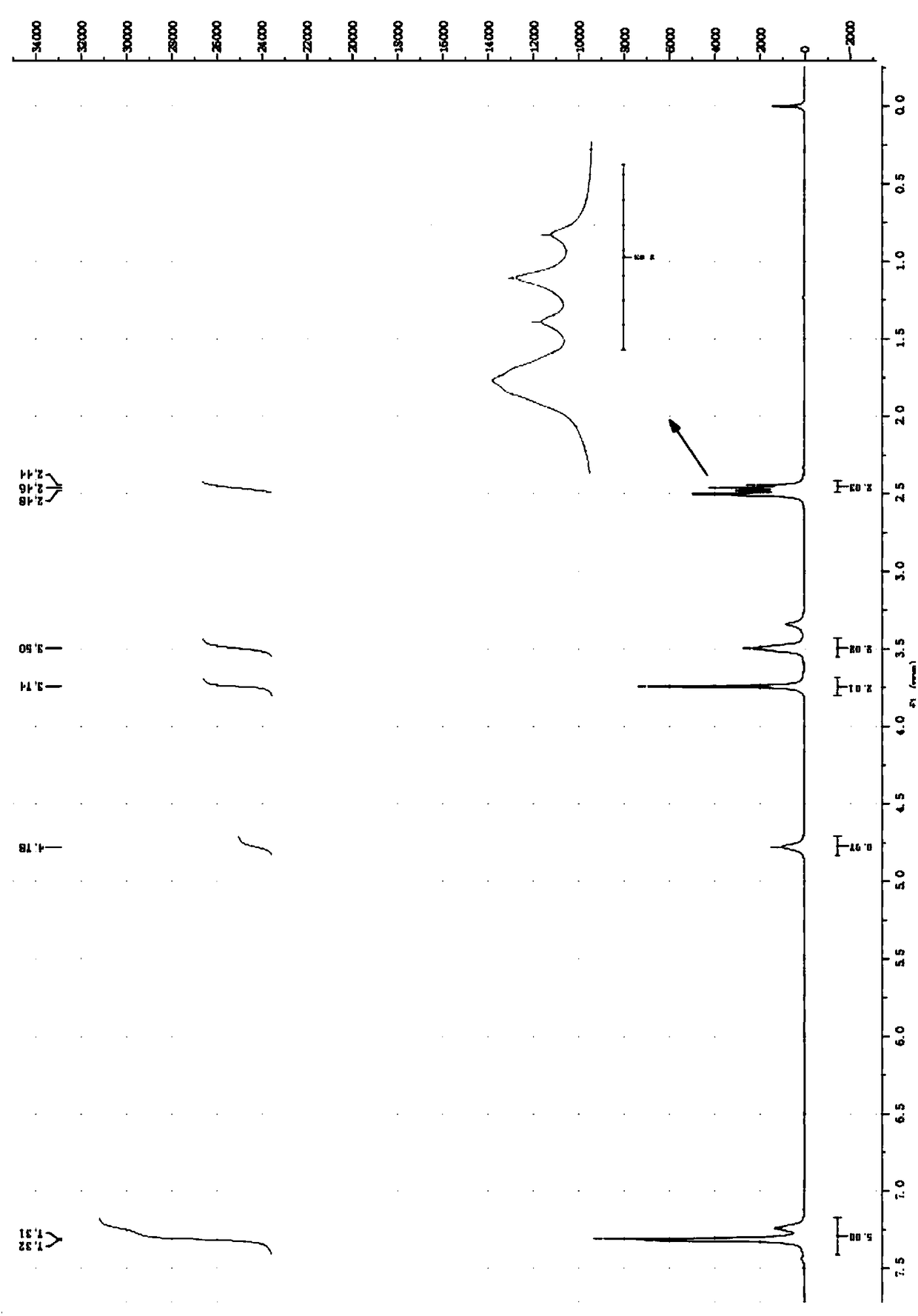
[0098] Transfer the oil phase obtained in Example 1 to the reactor, then add 7 parts of ethylamine aqueous solution (65-70% content) into the constant pressure dropping funnel below 20°C, heat up to 70°C, react for 1 hour, and cool to At room temperature, wash the reaction liquid with 50 parts of 8.3% sodium hydroxide solution, and separate the liquids to obtain the oil phase: O-isopropyl-N-ethylthiocarbamate with a purity of 96.7%, based on O- The yield of isopropyl-S-hydroxyethylxanthate is 85.7%, the aqueous phase is sodium 2-hydroxyethanethiolate, the purity is 28.097%, based on O-isopropyl-S-hydroxyethylxanthogen The yield of the ester was 98.1%, and 50 parts of hydrochloric acid solution with a concentration of 10.95% was added to the water phase, and acidified at room temperature for 2 hours to obtain the 2-mercaptoethanol product with a yield of 92.34%. The product was ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com