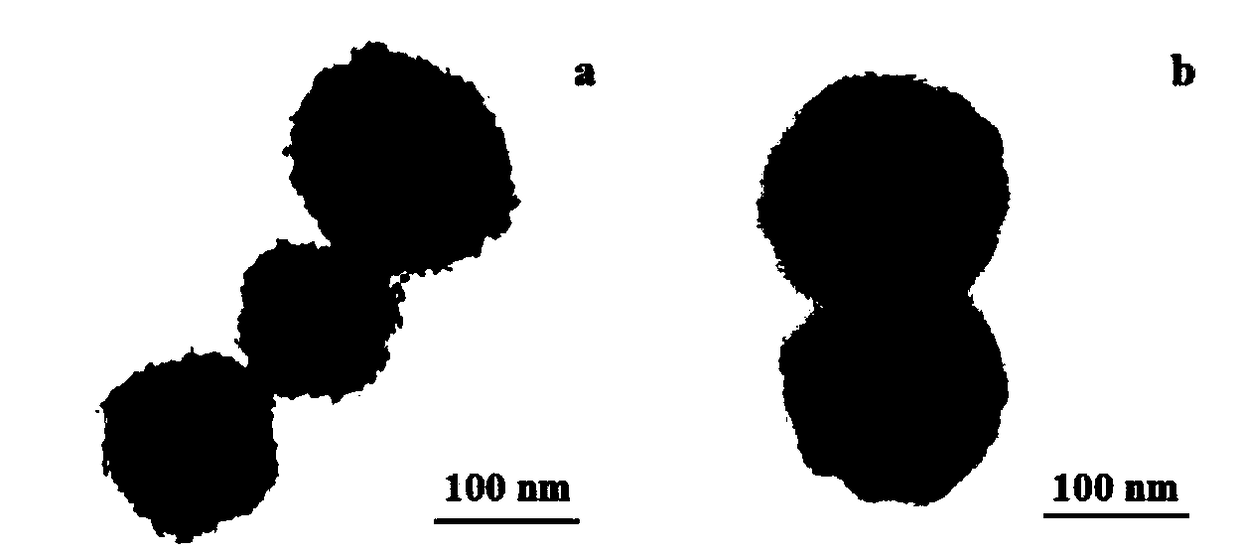
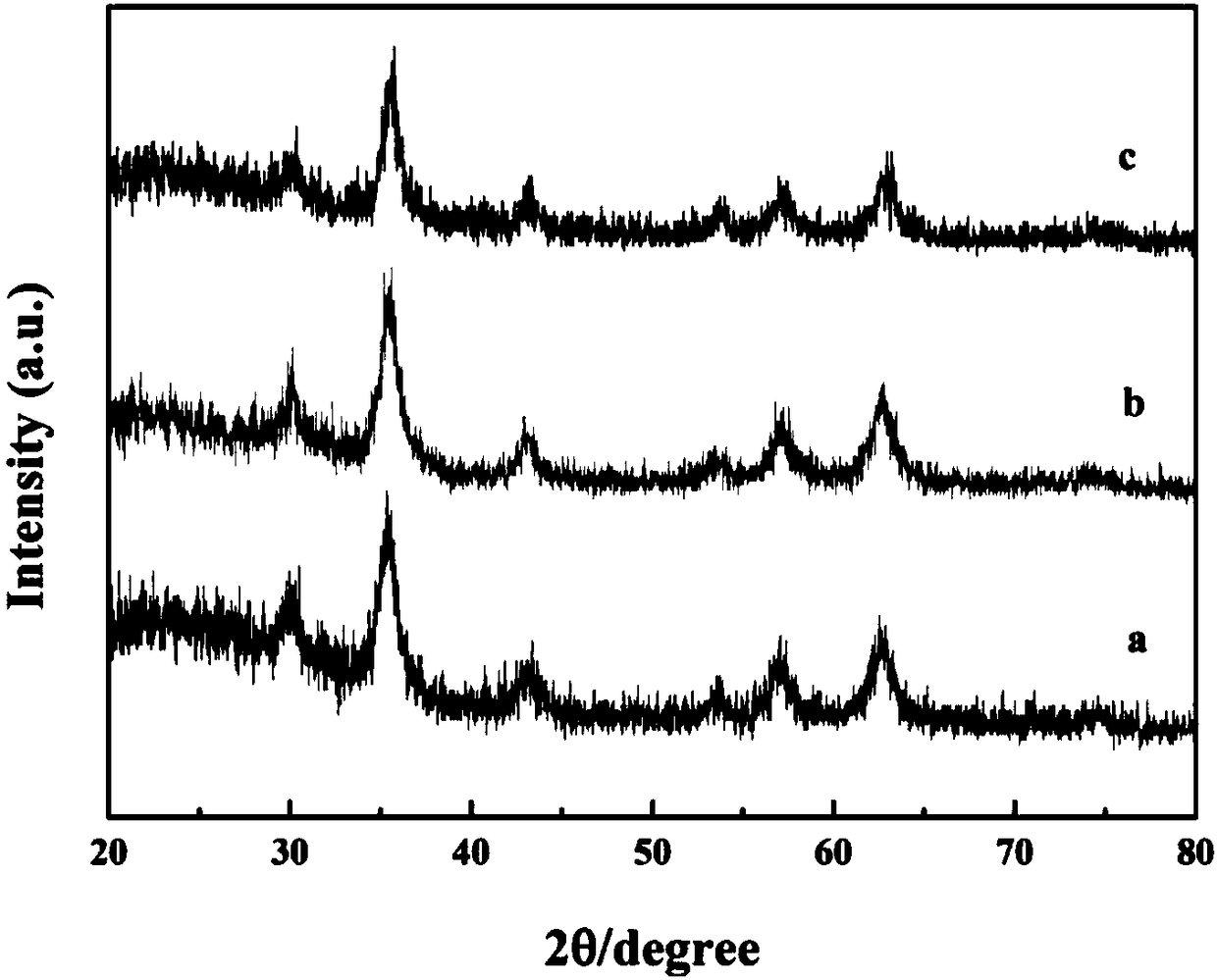
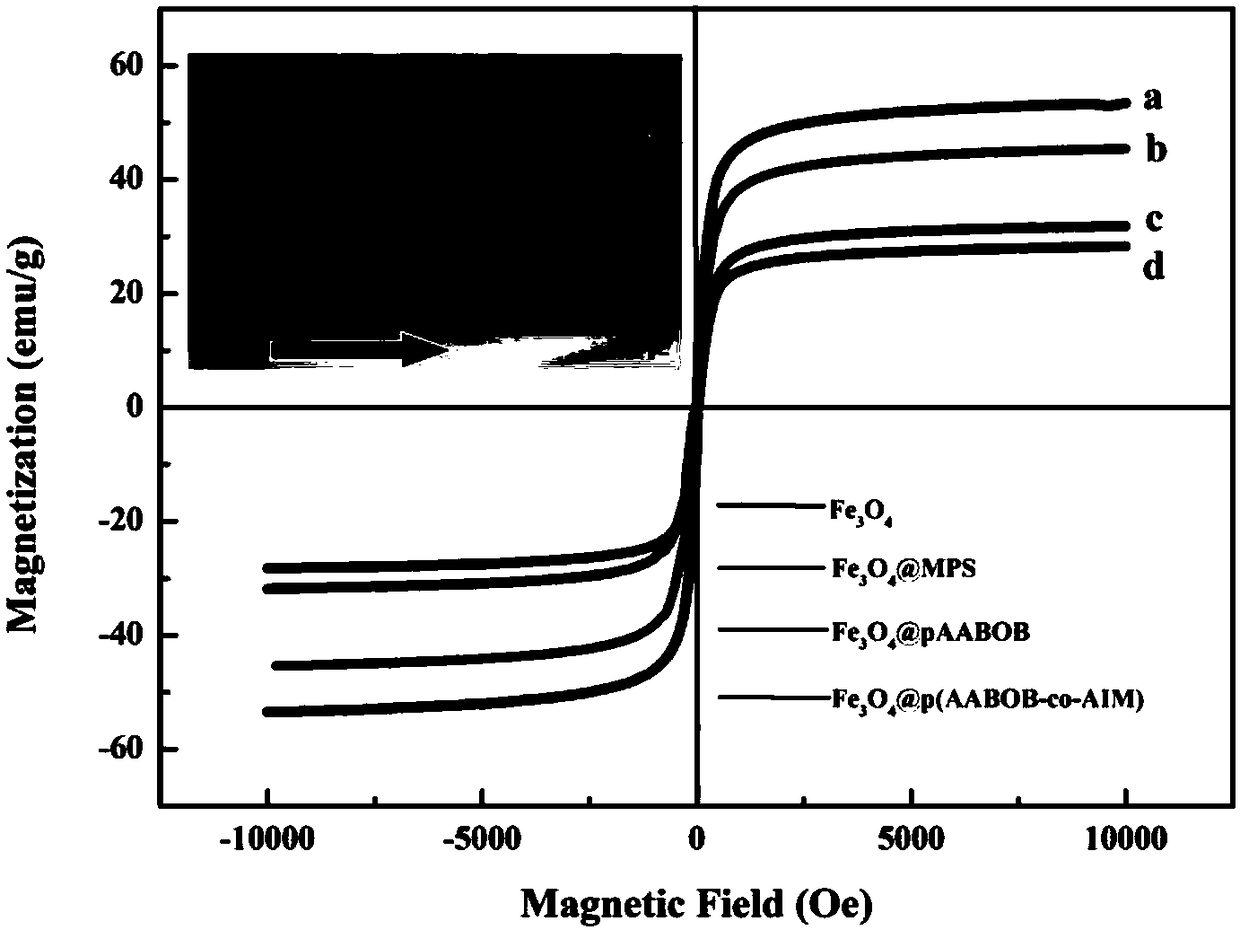
Wulff type phenylboronic acid (AABOB) functionalized magnetic nanometer microspheres and preparation method and applications thereof
A magnetic nano-type phenylboronic acid technology, applied in biochemical equipment and methods, chemical instruments and methods, nanotechnology and other directions, can solve the problems of poor reuse ability and low solid load, and achieve the effect of rapid separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1: Preparation of Fe3O4@p(AABOB-co-AIM) microspheres
[0073] (1) Synthesis of NBOB:
[0074] Add 5 mL of fuming nitric acid dropwise to 1 g of 2-(hydroxymethyl)phenylboronic acid cyclic monoester (BOB) at -40 °C while stirring, and continue stirring for 20 min after the addition is complete. After completion of the reaction as determined by TLC spot plate, the mixture was transferred to ice water and stirred vigorously for 1.5 h. After the reaction, the product was separated by suction filtration, washed several times with deionized water, and then vacuum-dried at 40° C. overnight.
[0075] (2) Synthesis of ABOB:
[0076] Dissolve 3 g of NBOB and 2 g (50%) Raney nickel in 90 mL of methanol, then add 5 mL of hydrazine hydrate to the mixture under nitrogen protection while stirring, and control the temperature of the system throughout the reaction process is 0°C. After the completion of the reaction was determined by TLC, the mixture was suction-filtered t...
Embodiment 2
[0085] Embodiment 2: Preparation of Fe3O4@p(AABOB-co-AIM) microspheres
[0086] (1) Synthesis of NBOB:
[0087] 15 mL of fuming nitric acid was added dropwise to 2.2 g of 2-(hydroxymethyl)phenylboronic acid cyclic monoester (BOB) at -40 °C while stirring, and the stirring reaction was continued for 20 min after the addition was completed. After completion of the reaction as determined by TLC spot plate, the mixture was transferred to ice water and stirred vigorously for 2.5 h. After the reaction, the product was separated by suction filtration, washed several times with deionized water, and then vacuum-dried at 40° C. overnight.
[0088] (2) Synthesis of ABOB:
[0089] Dissolve 3.8 g of NBOB and 4 g (50%) Raney nickel in 90 mL of methanol, then add 8.2 mL of hydrazine hydrate to the mixture under nitrogen protection, and control the temperature of the system during the entire reaction process is 0°C. After the completion of the reaction was determined by TLC, the mixture w...
Embodiment 3
[0098] Embodiment 3: Preparation of Fe3O4@p(AABOB-co-AIM) microspheres
[0099] (1) Synthesis of NBOB:
[0100] 10 mL of fuming nitric acid was added dropwise to 1.6 g of 2-(hydroxymethyl)phenylboronic acid cyclic monoester (BOB) at -40 °C while stirring, and the reaction was stirred for 20 min after the addition was completed. After completion of the reaction as determined by TLC spot plate, the mixture was transferred to ice water and stirred vigorously for 2 h. After the reaction, the product was separated by suction filtration, washed several times with deionized water, and then vacuum-dried at 40° C. overnight.
[0101] (2) Synthesis of ABOB:
[0102] Dissolve 3.4 g of NBOB and 3 g (50%) Raney nickel in 90 mL of methanol, then add 6.6 mL of hydrazine hydrate to the mixture under nitrogen protection while stirring, and control the temperature of the system throughout the reaction process is 0°C. After the completion of the reaction was determined by TLC, the mixture wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com