Construction of leucine dehydrogenase mutants and application thereof
A technology of leucine dehydrogenase and mutants, which can be applied in the application, enzyme, oxidoreductase and other directions, can solve the problems such as the influence of catalytic efficiency, and achieve the effect of efficient preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
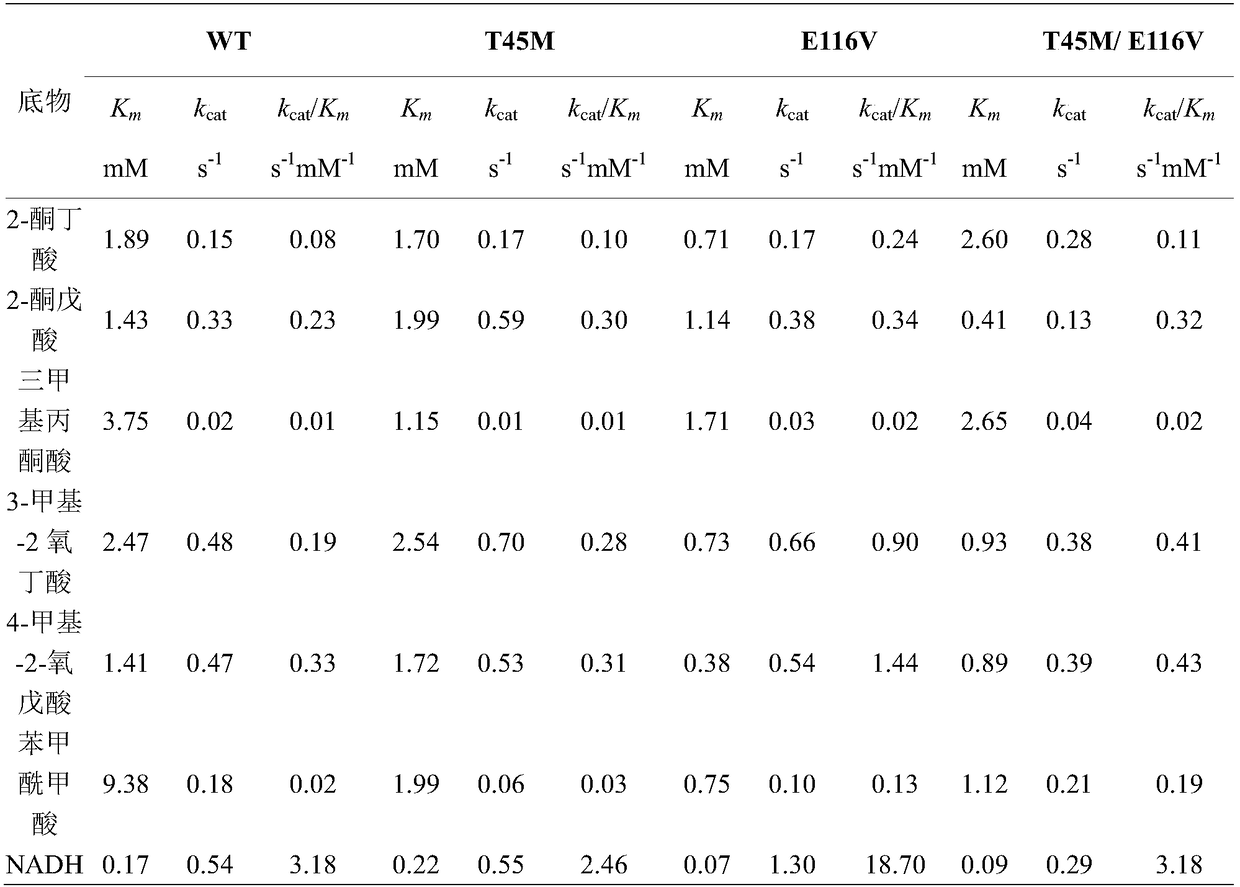
[0038] Example 1: Construction of Leucine Dehydrogenase Mutants
[0039] The pET-28a recombinant plasmid containing the leucine dehydrogenase gene derived from Bacillus cereus was used as a template.
[0040] The oligonucleotide fragment containing the mutation point is used as the upstream primer, and the oligonucleotide fragment near 2254 on the pET-28a plasmid is used as the downstream primer. The specific primers are as follows (bold and underlined are the mutation sites):
[0041] PF-T45M: 5'-CCGGCTCTTGGTGGA ATG AGAATGTGGACATAT-3'
[0042] PF-E116V: 5'-CGTTACATTACAGCT GTT GATGTTGGTACAACA-3'
[0043] PR-28a2254: 5'-GCCTTACTGGTTAGCAGAATG-3'
[0044] The mutant plasmid was constructed by two-step PCR method of the whole plasmid (Sanchis, J., Fernández, L., Carballeira, J.D., Drone, J., Gumulya, Y., & H., et al. Appl Microbiol Biotechnol, 2008, 81, 387-397.). PCR amplification system: template 0.5 μL, upstream and downstream primers 0.2 μL, dNTP Mix 2 μL, HS DNA Po...
Embodiment 2
[0046] Example 2: Induced expression of leucine dehydrogenase mutants
[0047] The leucine dehydrogenase mutant engineering bacteria constructed in Example 1 were inoculated into LB liquid medium containing 50 μg / mL kanamycin, cultured overnight at 37° C. and 160 r / min, and then transferred to 2 L of LB base. The inoculum size is 8%, the culture temperature is 37°C, the rotation speed is 300r / min, and the ventilation rate is 1.0vvm. After culturing for 2-3 hours, add IPTG with a final concentration of 0.5mM, and reduce the induction temperature to 28°C. After 16 hours of induction, collect the bacteria by centrifugation at 8,000 rpm at 4°C for 10 minutes, and store them in a -70°C refrigerator for later use.
Embodiment 3
[0048] Embodiment 3: Separation and purification of leucine dehydrogenase mutant
[0049] Take 0.5 g of the wet bacterial cells collected in Example 2, wash twice with 10 mL of 50 mM PB buffer solution of pH 7.5, resuspend in 10 mL of 50 mM PB buffer solution of pH 7.5, oscillate and shake, and then crush under ultrasonic waves. Break for 1s, stop for 3s, the total time is 15min. The cell lysate was centrifuged at 12,000 rpm for 20 min to remove cell debris, and the supernatant, namely the crude enzyme solution, was collected and filtered with a 0.22 μm filter membrane for subsequent separation and purification of the enzyme. The purification column is a Ni-NTA column with a packing volume of 5mL. First equilibrate the Ni-NTA column with loading equilibration buffer M20 (20mM sodium phosphate, 500mM NaCl and 20mM imidazole, pH 7.4), and load the column at a rate of 0.5mL / min. Sample crude enzyme solution, eluted with loading equilibration buffer M20 to remove unadsorbed prote...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com