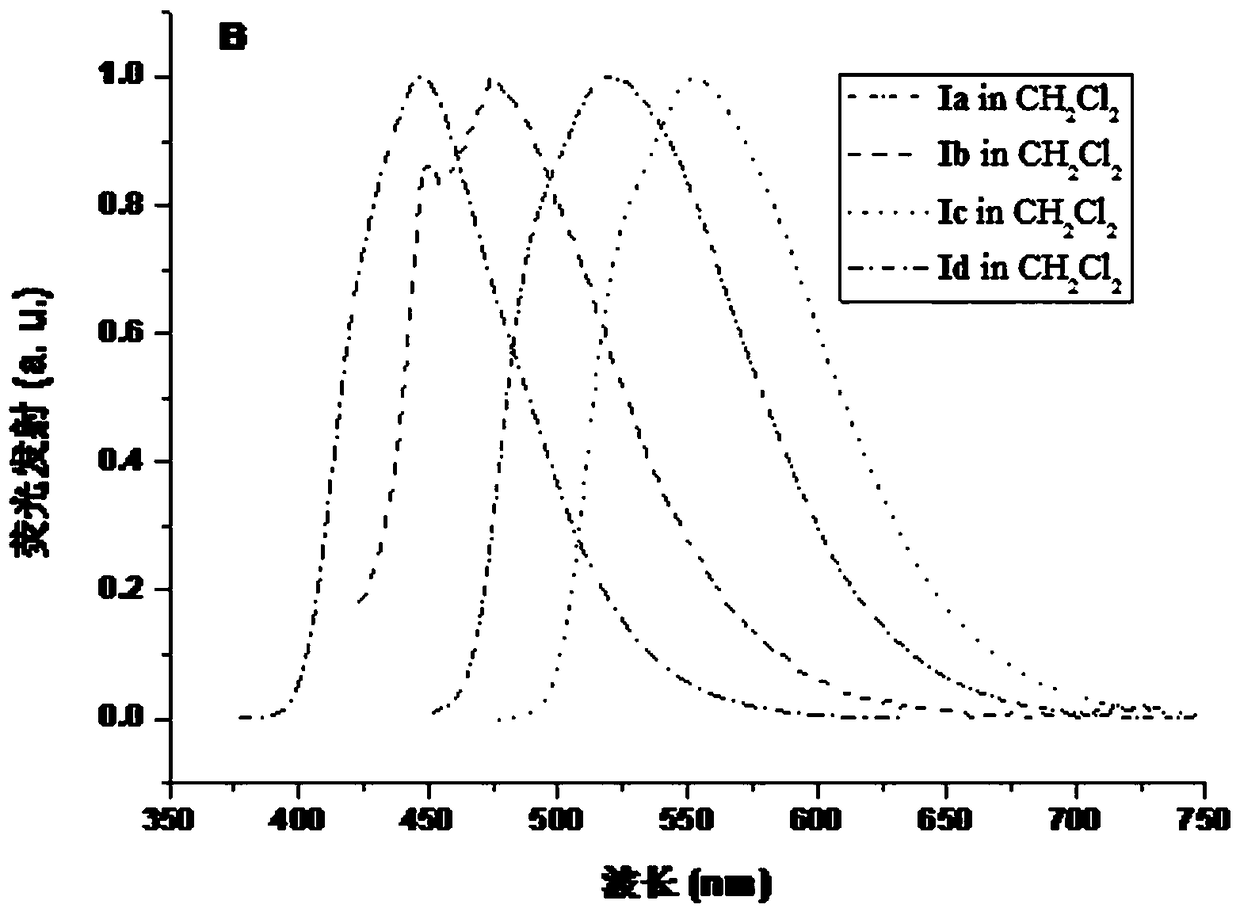
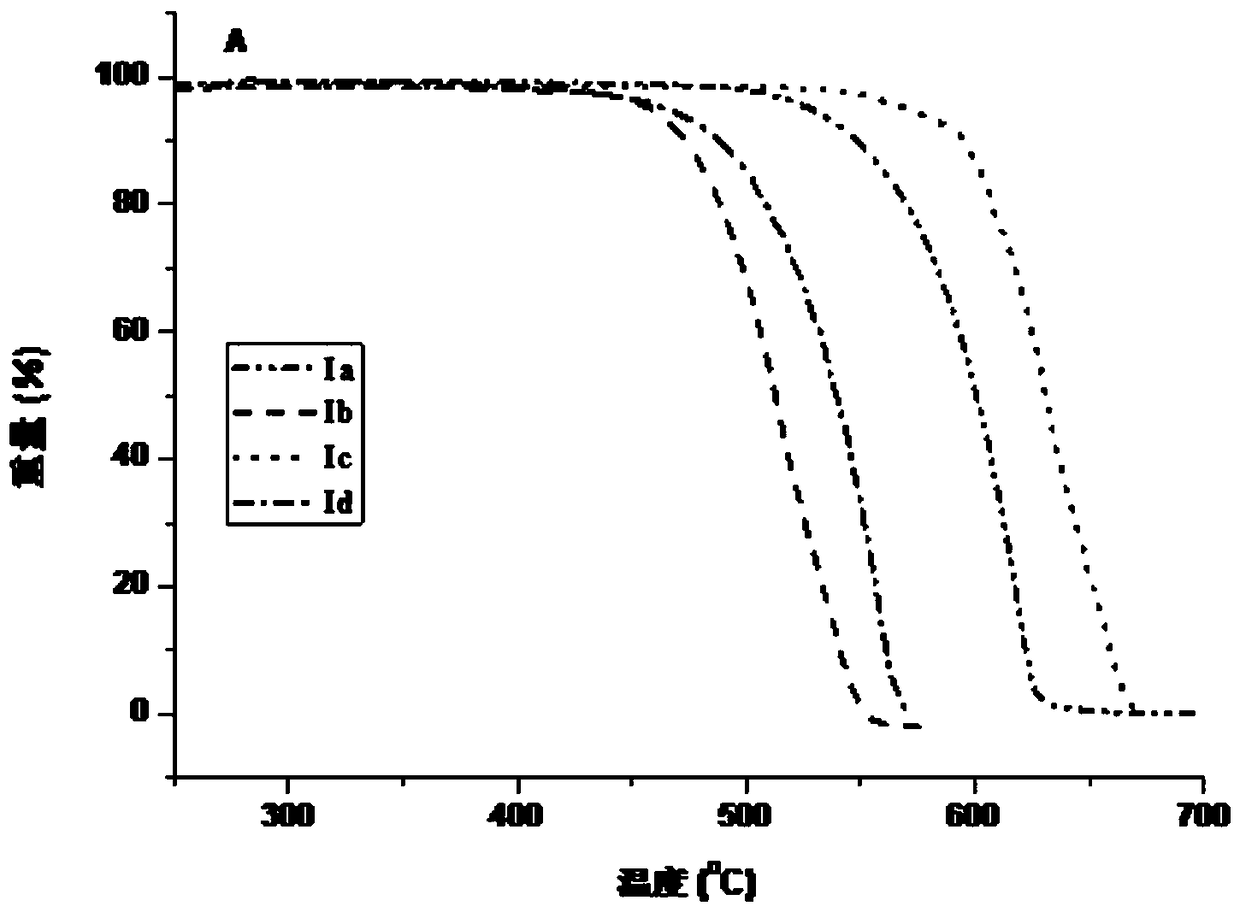
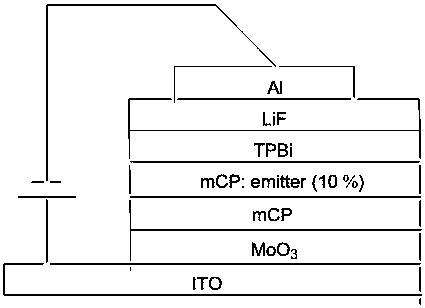
Organic electroluminescence compound with diphosphopentadiene condensed ring and synthesis method of compound
A technology of heterocyclopentadiene and compound, which is applied in the field of organic electroluminescent materials to achieve the effect of reducing LUMO orbital, improving electron injection and transmission capability, and simple route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The synthesis of embodiment 1 compound Ia
[0027] (1) Synthesis of compound III
[0028]
[0029] Under nitrogen protection, add 4.85g of compound II, 2.50g of trimethylsilylacetylene, 50ml of triethylamine, 100mg of cuprous iodide, 150mg of dichlorobis(triphenylphosphine)palladium, close the reaction system, and stir overnight at room temperature. The reaction process was detected by gas phase. After the reaction was completed, triethylamine was distilled off under reduced pressure, and the column was eluted with n-hexane eluent, and spin-dried to obtain the crude product. The pure product was obtained by silica gel column chromatography, 3.2 g of white solid, and the yield was 80%. .
[0030] 1 H NMR (300MHz, CDCl 3 )δ=0.08(s,18H),7.65(s,2H).
[0031] (2) Synthesis of compound IV
[0032]
[0033] Under the protection of nitrogen, add 4.25g of compound III, then add 20ml of anhydrous tetrahydrofuran, 20ml of anhydrous ether, cool the system to -75°C ~ -80°...
Embodiment 2
[0047] The synthesis of embodiment 2 compound 1b
[0048]
[0049] Under the protection of nitrogen, add 3.7g of compound V to the reaction flask, then add 6.2g of compound VIb, then add 20ml of dichloroethane, 2.5g of silver hexafluorophosphate, 5g of o-tetrachlorobenzoquinone, 0.2g of palladium acetate, 70-80 ℃ reflux reaction overnight, and the reaction system was processed to obtain the final product Compound I. The pure product was obtained by silica gel column chromatography as a green solid. The cis structure was 5.9g, and the yield was 85%, and the trans structure was 5.7g, and the yield was 82%.
[0050] Shun 31 P{1H}NMR (121MHz, CDCl 3 )δ=40.7; 1 H NMR (300MHz, CDCl 3 )δ=6.90-7.16(m,4H),7.22-7.37(m,4H),7.50-7.78(m,16H).
[0051] opposite 31 P{1H}NMR (121MHz, CDCl 3 )δ=39.1; 1 H NMR (300MHz, CDCl 3 )δ=6.80-7.06(m,4H),7.25-7.39(m,4H),7.45-7.50(m,10H),7.50-7.78(m,6H).
[0052] VIb was synthesized by the method of Via compound, replacing 2,2'-dibromobipheny...
Embodiment 3
[0053] The synthesis of embodiment 3 compound Ic
[0054]
[0055] Add 6.7g of compound Ia (cis) to the reaction flask, then add 20ml of anhydrous toluene, 15.0g of Lawson's reagent, react overnight at 85-95°C, and obtain pure product by silica gel column chromatography, yellow solid cis 5.8g, yield 83 %, trans 6.3g, yield 89%.
[0056] Shun 31 P{1H}NMR (121MHz, CDCl 3 )δ=45.0; 1 H NMR (300MHz, CDCl 3 )δ=7.30-7.52(m,6H),7.70-7.72(m,2H),7.67-7.83(m,12H),8.19-8.22(m,4H),8.78-8.92(m,4H).
[0057] opposite 31 P{1H}NMR (121MHz, CDCl 3 )δ=45.9; 1 H NMR (300MHz, CDCl 3 )δ=7.23-7.30(m,2H),7.31-7.53(m,4H),7.67-7.78(m,14H),8.19-8.22(m,4H),8.80-8.95(m,4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com