Preparation method of magnetic ferric alginate mesoporous carbon microsphere capable of adsorbing heavy metal elements
A technology for adsorbing heavy metals and alginic acid, which is applied in the fields of alkali metal compounds, chemical instruments and methods, and adsorption water/sewage treatment. It can solve the problems of poor stability of adsorption materials, difficult recovery of heavy metals, and high processing costs, and achieve good thermal stability. , Improve recycling rate, good adsorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
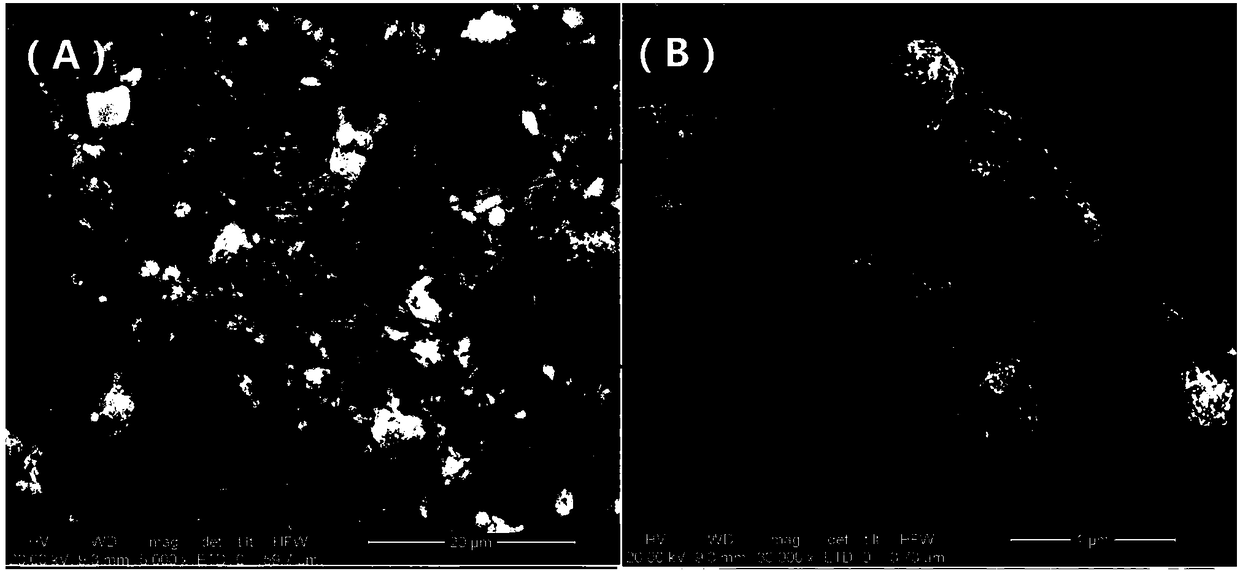
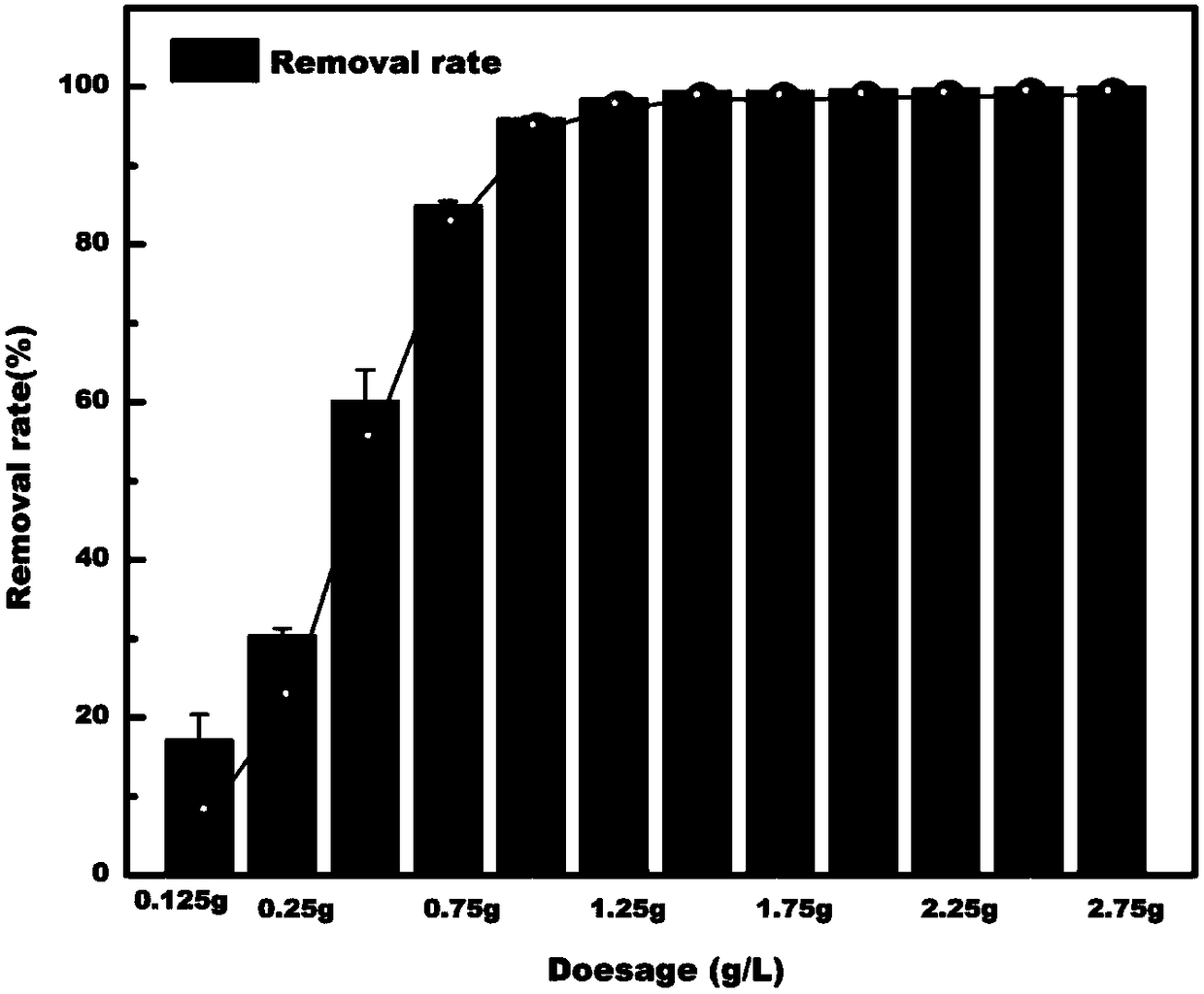
Image
Examples
Embodiment 1
[0025] A preparation method of magnetic iron alginate mesoporous carbon microspheres capable of adsorbing heavy metal elements, comprising the following steps:
[0026] S1), using a ten-thousand-digit balance, weigh 2g of chemically pure grade (viscosity (10g / L) ≥ 0.02Pa·s) sodium alginate powder and dissolve it in 98mL of deionized water under hot bath conditions to prepare a 2% sodium alginate saturated solution , ready to use after cooling;
[0027] S2), add 2 g of urea to the sodium alginate saturated solution, stir magnetically for 30 minutes and then cool to obtain the SAC precursor solution for use;
[0028] S3), ferric nitrate nonahydrate solid powder is dissolved in deionized water, configured into a 5% ferric nitrate solution, and then the SAC precursor solution is added dropwise to the ferric nitrate solution at a rate of 20 drops / min under stirring conditions, When the SAC precursor solution encounters the ferric nitrate solution, small droplets in the shape of wa...
Embodiment 2
[0033] A preparation method of magnetic iron alginate mesoporous carbon microspheres capable of adsorbing heavy metal elements, comprising the following steps:
[0034] S1), using a ten-thousand-digit balance, weigh 3g of sodium alginate powder of chemical purity (viscosity (10g / L) ≥ 0.02Pa·s) and dissolve it in 97mL of deionized water under hot bath conditions to form 3% saturated sodium alginate Solution, stand-by after cooling;
[0035] S2), add 3 g of urea to the sodium alginate saturated solution, stir magnetically for 30 minutes and then cool to obtain the SAC precursor solution for use;
[0036] S3), ferric nitrate nonahydrate solid powder is dissolved in deionized water, configured into a 5% ferric nitrate solution, and then the SAC precursor solution is added dropwise to the ferric nitrate solution at a rate of 20 drops / min under stirring conditions, When the SAC precursor solution encounters the ferric nitrate solution, small droplets in the shape of water will be f...
Embodiment 3
[0041] A preparation method of magnetic iron alginate mesoporous carbon microspheres capable of adsorbing heavy metal elements, comprising the following steps:
[0042] S1), using a ten-thousand-digit balance, weigh 3g of sodium alginate powder of chemical purity (viscosity (10g / L) ≥ 0.02Pa·s) and dissolve it in 97mL of deionized water under hot bath conditions to form 3% saturated sodium alginate Solution, stand-by after cooling;
[0043] S2), adding 3 g of urea in parts by mass to the saturated sodium alginate solution, stirring with magnetic force for 30 minutes, cooling to obtain the SAC precursor solution for use;
[0044] S3), ferric nitrate nonahydrate solid powder is dissolved in deionized water, configured into a 10% ferric nitrate solution, and then the SAC precursor solution is added dropwise to the ferric nitrate solution at a rate of 20 drops / min under stirring conditions, When the SAC precursor solution encounters the ferric nitrate solution, small droplets in t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com