Methyl gallate analogue and application thereof
A technology of methyl gallate and analogs, applied in the field of methyl gallate analogs and their synthesis, can solve the problems of unclear anti-inflammatory targets, limited toxic and side effects, and long drug cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
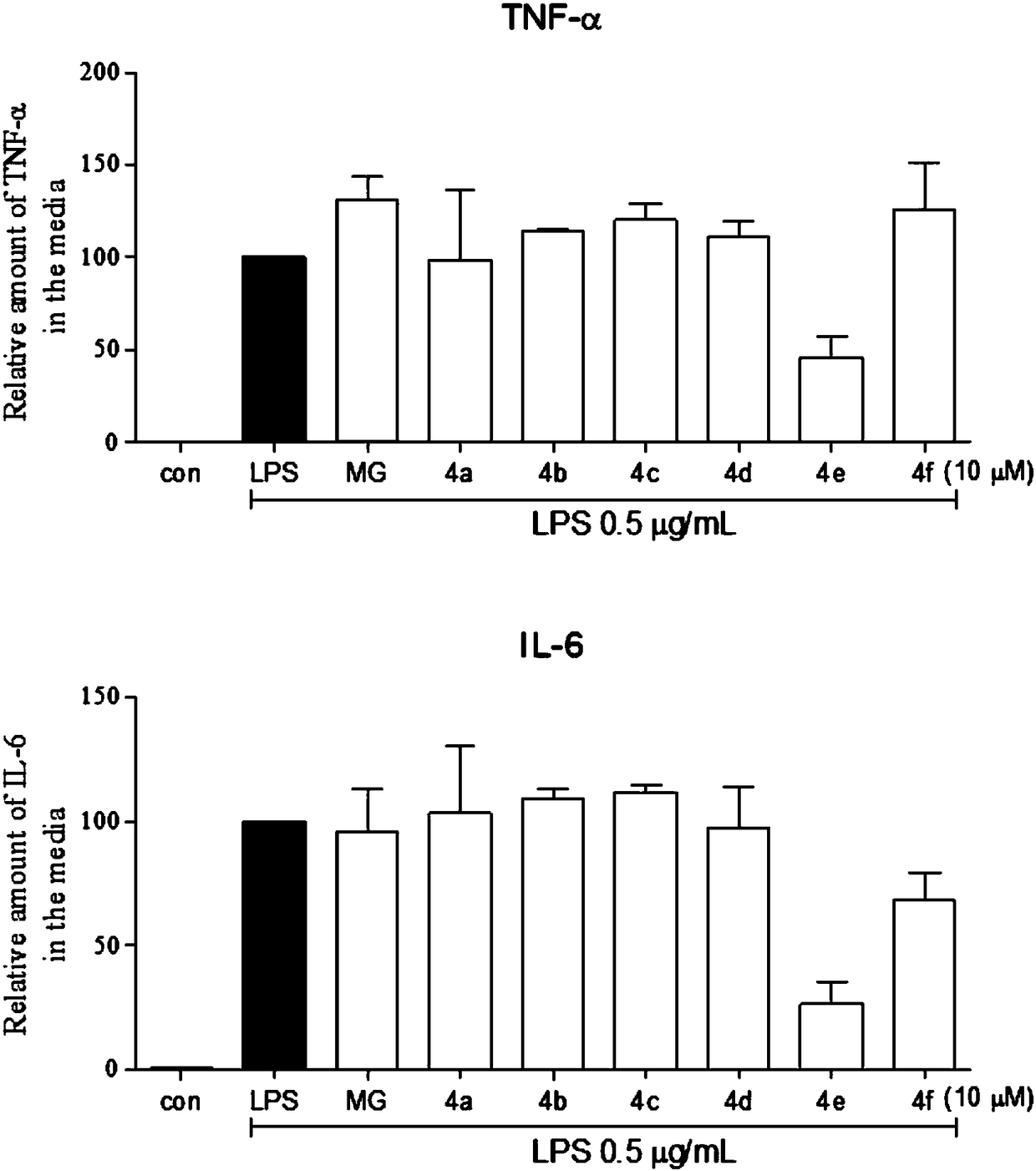
Image
Examples
Embodiment 1
[0025] The synthesis of embodiment 1 intermediate 2
[0026] Add 3,4,5-trihydroxybenzoic acid (0.5g, 2.94mmol), 20mL tetrahydrofuran, 3.66g potassium carbonate into a 50mL round-bottomed flask, slowly add 4.19mL benzyl bromide dropwise under nitrogen protection at 40°C, and dropwise , reacted at a constant temperature of 40° C. for 12 hours, and added 20 mL of water to the reaction liquid to terminate the reaction. Extract three times with chloroform, 20 mL each time, separate the organic phase, wash twice with water, dry with anhydrous MgSO4, evaporate the solvent on a rotary evaporator under reduced pressure, and dissolve the residue in 50% ethanol containing 5 mol / L sodium hydroxide The solution was refluxed at room temperature for 12 hours in 40 mL of the solution. After cooling, the pH value was adjusted to 2 with dilute hydrochloric acid. After precipitation, the white solid 2 was obtained by filtration, and the next step of reaction could be carried out directly without...
Embodiment 2
[0027] The synthesis of embodiment 2 intermediate 3a-3f
[0028] Taking the synthesis of 3a as an example, the specific operation is as follows: add 3,4,5-tribenzyloxybenzoic acid (0.3 g, 0.7 mol), 2-(7-benzotriazole oxide) into a 50 mL round bottom flask -N,N,N',N'-tetramethyluronium hexafluorophosphate (0.26g, 0.7mol), 0.2mLN,N'-diisopropylethylamine and 10mL dichloromethane, stirred at room temperature and refluxed for 20 Minutes later, ethylpropylamine (0.06 g, 0.7 mol) was slowly added dropwise from the dropping funnel. After the dropwise addition, stirring was continued at room temperature for 5 hours, and 20 mL of water was added to the reaction solution to terminate the reaction. Extract 2 times with ethyl acetate, 30 mL each time, separate the organic phase, wash 2 times with water, anhydrous MgSO 4 After drying, the solvent was evaporated under reduced pressure on a rotary evaporator, and the residue was recrystallized with methanol-water (1:1) solvent system to obt...
Embodiment 3
[0029] Synthesis of embodiment 3 target compound 4a-4f
[0030] Taking the synthesis of 4a as an example, the specific operation is as follows: in a 50mL round bottom flask, add the previous step product 3a (0.29g, 0.6mmol), 12mL of methanol-water (1:1v / v) and a catalytic amount of 10% palladium carbon ( 0.1 mmol), hydrogen was introduced under stirring conditions, and the reaction was carried out at room temperature for 1 hour, and the reaction solution was filtered under reduced pressure. Evaporate methanol under reduced pressure on a rotary evaporator, slowly add dichloromethane dropwise to the raffinate until all precipitates are precipitated, filter to obtain a gray solid which is the target compound, 0.04g, and the yield of compound 4a-4f is 33.3-46.6 %between.
[0031] Product characterization data are as follows:
[0032] 3,4,5-Trihydroxy-N-propylbenzamide (4a)
[0033] Gyay powder, 33.3% yield.m.p: 143.8-146.2℃. 1 H NMR (500MHz, DMSO-d 6 )δ:9.02(2H,s,Ar-OH),8.65(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com