Method for representing lithium ion concentration change in electrolyte during cyclic process
A technology of cyclic process and concentration change, applied in the direction of measuring electrical variables, electrochemical generators, electrochemical variables of materials, etc., can solve problems such as fluctuations, lack of intuitive understanding, and limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
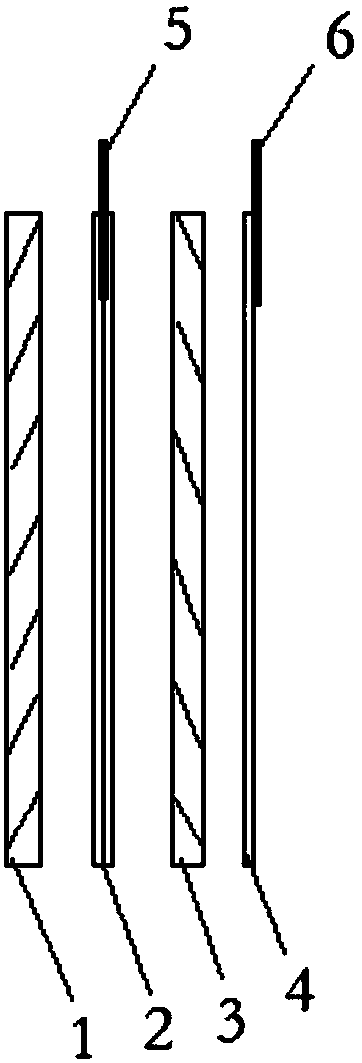
[0030] Such as figure 1 As shown, the lithium-ion battery includes a positive electrode sheet 1 and a negative electrode sheet 3, a double-layer separator 2 is provided between the positive electrode sheet 1 and the negative electrode sheet 3, and a first reference electrode 5 is provided between the double-layer separator 2; A single-layer separator 4 is arranged on the outside of the negative electrode sheet 3 , and a second reference electrode 6 is arranged on the outside of the single-layer separator 4 .
[0031] Both the first reference electrode 5 and the second reference electrode 6 are copper wires.
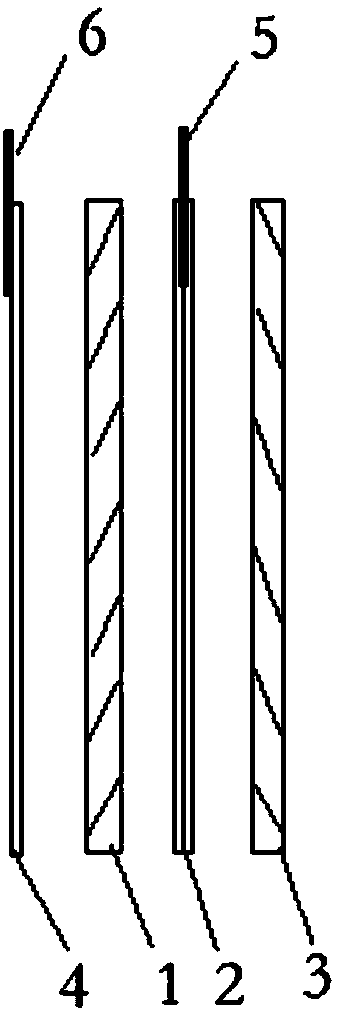
[0032] Such as figure 2 as shown in figure 1 As shown, the lithium-ion battery includes a positive electrode sheet 1 and a negative electrode sheet 3, a double-layer separator 2 is provided between the positive electrode sheet 1 and the negative electrode sheet 3, and a first reference electrode 5 is provided between the double-layer separator 2; A single-layer separa...
Embodiment 2
[0035] (1) Assemble according to the first structure in Example 1, and assemble the lithium ion battery with two reference electrodes in a dry room by injecting liquid into the seal;
[0036] (2) Put the two reference electrodes in the lithium-ion battery on the Xinwei channel battery comprehensive performance tester respectively, and respectively carry out the positive and negative pole pieces to pass through lithium for 240min under the current condition of 0.02mA; make the two reference electrodes (copper Silk) uniformly adhered to a layer of metal lithium on the surface;
[0037] (3) The lithium-ion battery after step (2) is processed into two weeks at a rate of 0.05C;
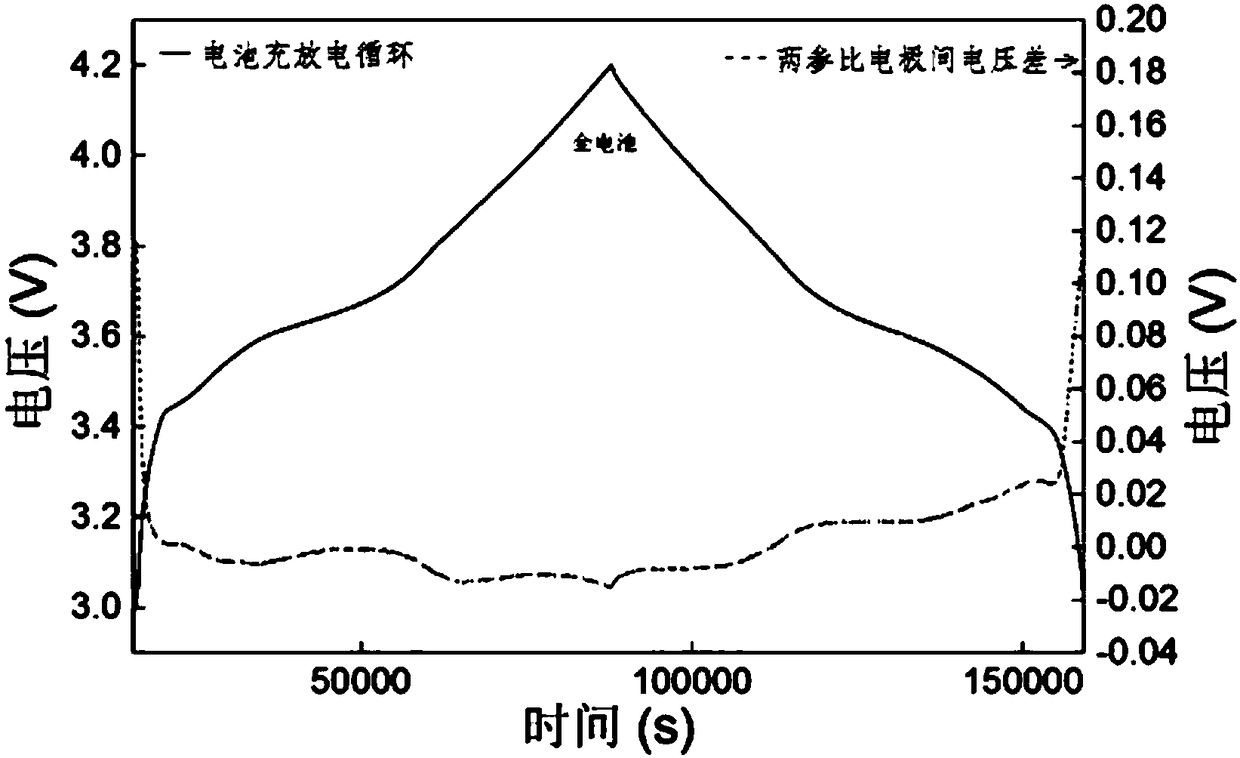
[0038] (4) Connect the lithium-ion battery after the above-mentioned formation to the Xinwei test workstation, set the constant current charge and discharge at 0.05C, 0.1C, 0.5C, and 1C for 2 weeks, and use a multi-channel recorder to monitor the negative electrodes respectively. Voltage E1, E2 to two ref...
Embodiment 3
[0042] (1) Assemble according to the first structure in Example 1, and assemble the lithium ion battery with two reference electrodes in a dry room by injecting liquid into the seal;
[0043] (2) Put the two reference electrodes in the lithium-ion battery on the Xinwei channel battery comprehensive performance tester respectively, and respectively carry out the positive and negative pole pieces to pass through lithium for 240min under the current condition of 0.02mA; make the two reference electrodes (copper Silk) uniformly adhered to a layer of metal lithium on the surface;
[0044] (3) The lithium-ion battery after step (2) is processed into two weeks at a rate of 0.05C;
[0045] (4) Connect the lithium-ion battery after the above-mentioned formation to Xinwei test workstation, set 0.05C constant current charge and discharge for 1 week, 0.1C, and 1C rate for 2 weeks, and monitor them separately with a multi-channel recorder The voltages E1 and E2 of the negative electrode t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com