Pharmaceutical or nutritional combination comprising beta-hydroxy-betamethylbutyrate
A technology of methyl butyrate and nutrition, which is applied in the direction of drug combination, drug delivery, active ingredients of anhydride/acid/halide, etc. It can solve the problems of HMB difficult to handle, reduce manufacturing efficiency, and difficult to prepare the composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0062]According to exemplary embodiments, multiple proteins, including one protein or more than one protein, can be used to provide a source of protein in a nutritional combination. Proteins suitable for use in nutritional compositions according to embodiments disclosed herein include, but are not limited to, hydrolyzed, partially hydrolyzed or non-hydrolyzed proteins or protein sources, and may be derived from any known or otherwise suitable source, such as milk (eg, milk). , casein, whey), animals (eg, meat, fish), grains (eg, rice, corn), vegetables (eg, soybeans, peas, potatoes), or combinations thereof.
[0063] Non-limiting examples of such protein sources include whey protein concentrate, whey protein isolate, whey protein hydrolyzate, acid casein, sodium caseinate, calcium caseinate, potassium caseinate, casein hydrolysate milk protein concentrate, milk protein isolate, milk protein hydrolyzate, skim milk, low-fat milk, nonfat dry milk, skim milk powder, skim milk conc...
Embodiment 1
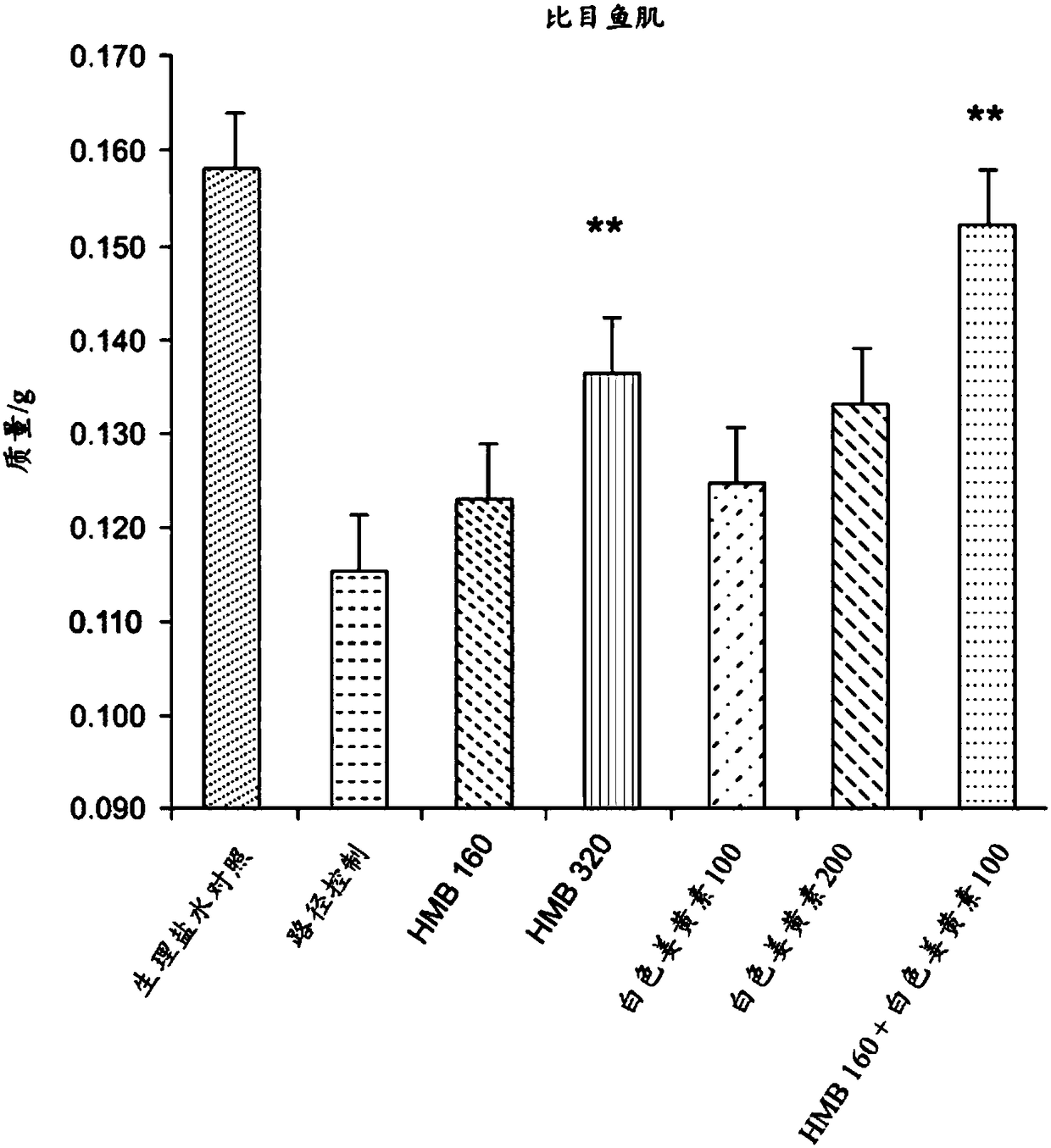
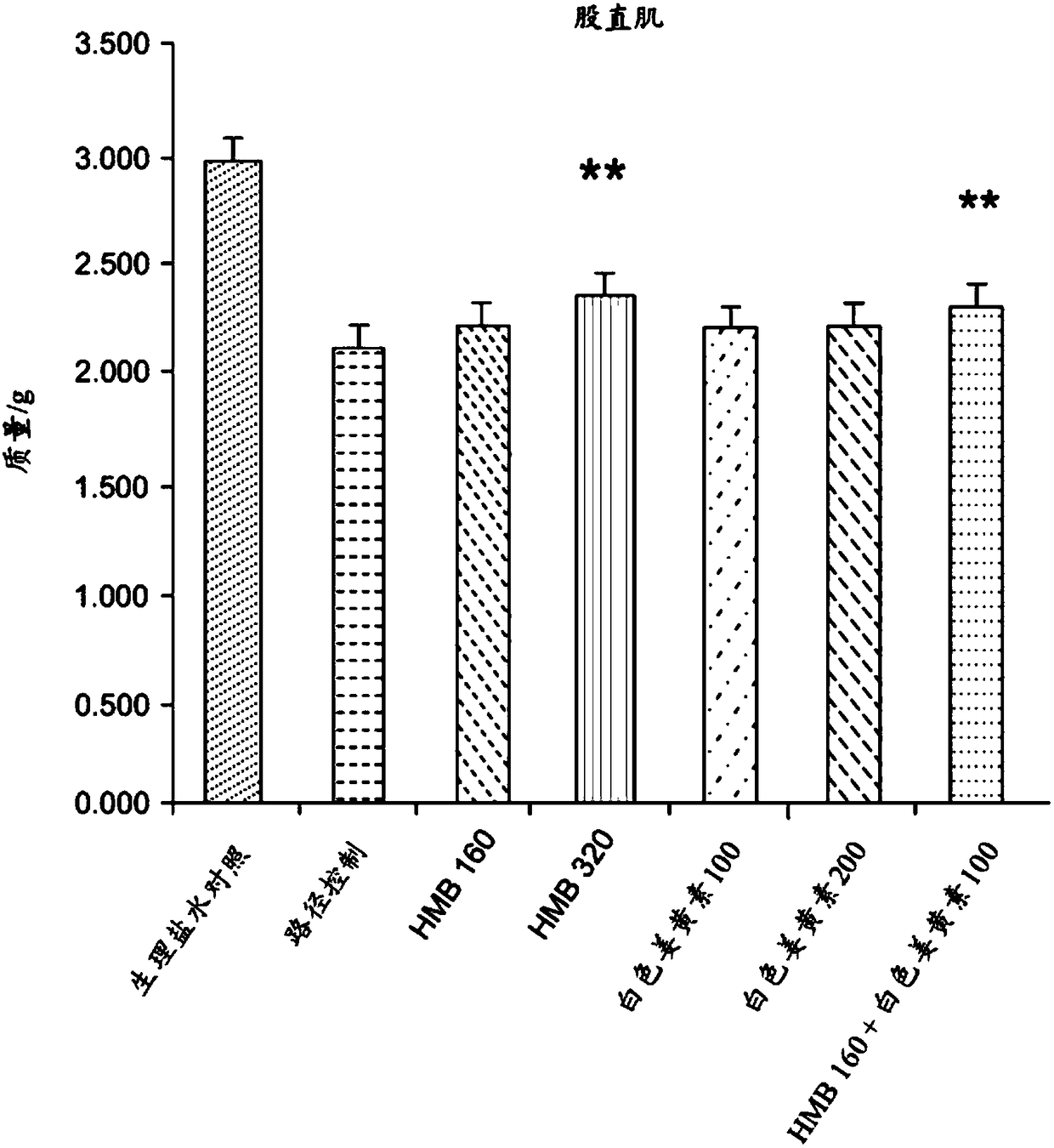
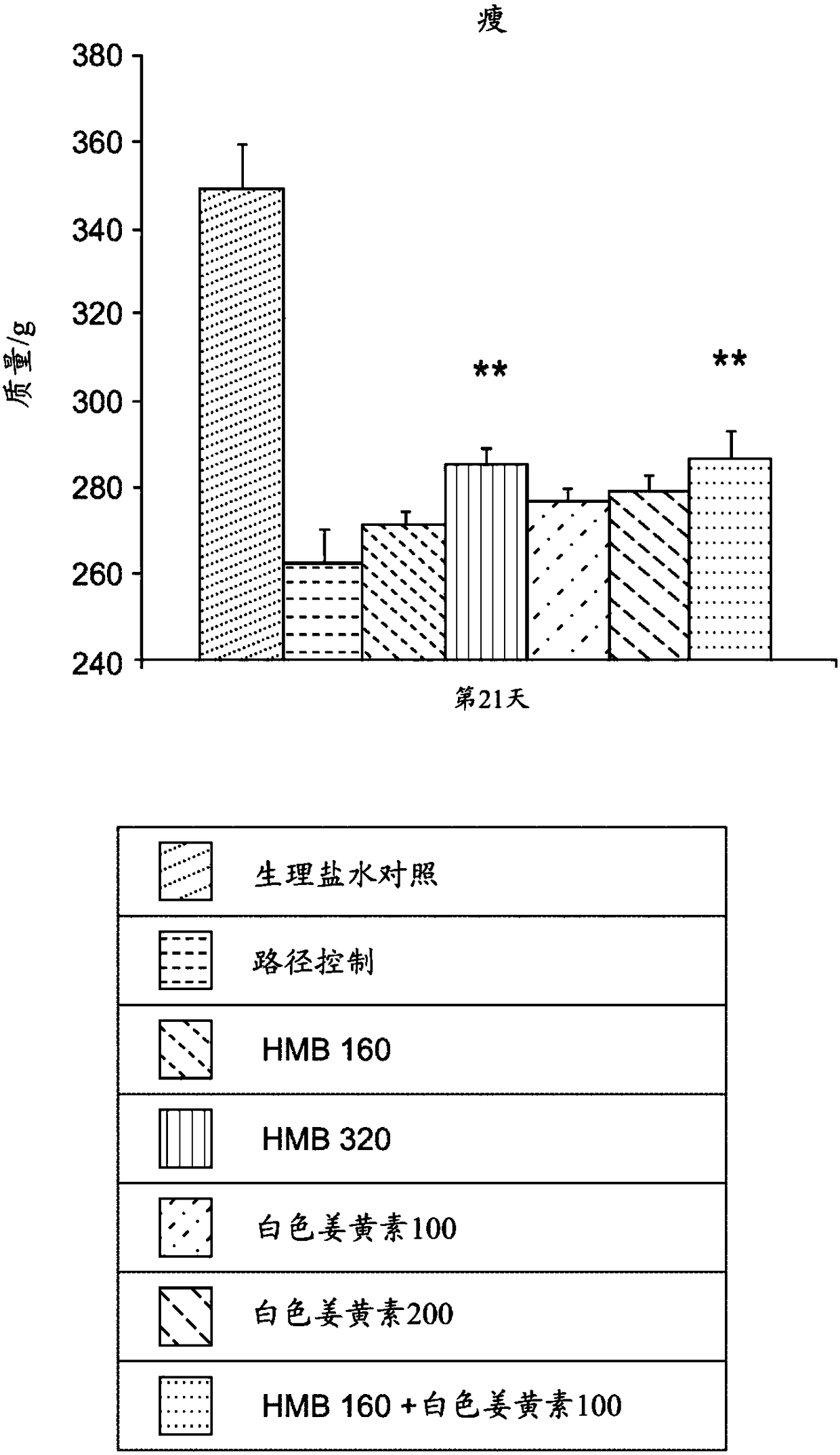
[0148] A study was conducted to investigate the effect of a composition containing HMB and white curcumin (THC) in a well-validated animal model of human muscle loss (β-Hydroxy-β-methylbutyrate (HMB) normalizes dexamethasone-induced autophagy-lysosomal pathway in skeletal muscle. Giron MD, Vilchez JD, Shreeram S, Salto R, Manzano M, Cabrera E, Campos N, Edens NK, RuedaR, Lopez-Pedrosa JM. PLoS One. 2015 Feb 6;10(2):e0117520 .doi:10.1371 / journal.pone.0117520.eCollection2015).
[0149] Experimental Design - Male Sprague-Dawley rats (8-10 weeks old) (n=42) weighing 270-310 g were randomly assigned to seven experimental groups (six rats per group). Groups 1-6 were administered dexamethasone (a synthetic glucocorticoid) at 0.1 mg / kg / day by intraperitoneal injection for 21 days to induce muscle loss. The dexamethasone was administered at 2 mL / kg of 1.75 mg dexamethasone / 35 mL normal saline. Group 7 (sham control) was administered saline without dexamethasone.
[0150] Group 1 (pa...
Embodiment 2
[0162] Example 2 illustrates a nutritional powder according to the present disclosure, the ingredients of which are listed in the table below. The product is prepared by spray drying method. All ingredients are listed in kg / 1000kg bulk product unless otherwise specified. Comparison with known HMB-containing nutritional powders.
[0163]
[0164]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com