Nucleic acid molecule and application to humanized antibody
A nucleic acid molecule and nucleotide sequence technology, applied in the field of nucleic acid molecules and their applications, can solve the problems of the small number of B-cells in the early stage, and the specificity and diversity of antibodies need to be improved, so as to reduce artificial modification and improve druggability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0061] Transfer the modified human immunoglobulin gene heavy chain vector into mice, and then immunize transgenic mice containing human immunoglobulin genes to obtain fully human antibodies. The specific steps are as follows:
[0062] 1. Construction of Immunoglobulin Gene Vectors
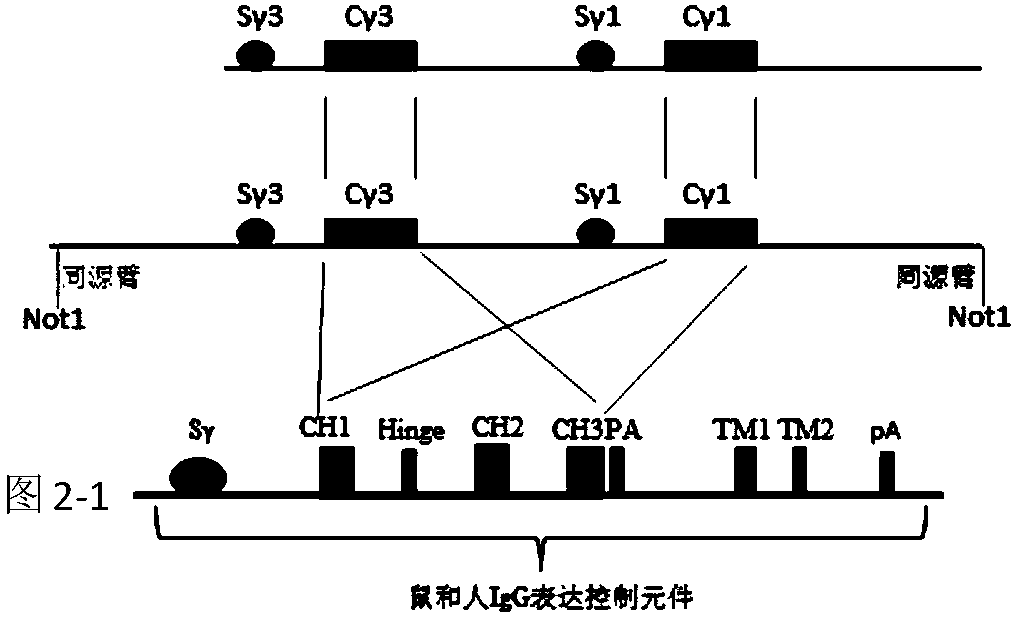
[0063] 1) Construction of immunoglobulin heavy chain gene (such as Figure 5 )
[0064] First, use PCR and gene synthesis to obtain the 5'-enhancer sequence of mouse IgM, all expression regulatory sequences of IgM and homology arms and screening genes (such as figure 1 ). Re-obtain human Igγ3 and Igγ1 and 3'-LCR sequences as well as homology arms and screening genes (such as image 3 ). Then all DNA fragments are homologously recombined to obtain new DNA nucleic acid molecules, and then the above-mentioned transformed DNA fragments are transferred to YAC or BAC vectors containing human immunoglobulin heavy chain genes (Ig) to construct heavy immunoglobulins. Gene clusters of chain genes such ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com