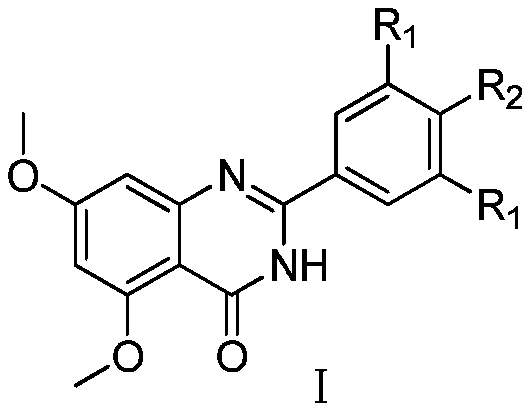
Derivative of RVX-208 based on BRD4 inhibitor as well as preparation method and application of derivative
An RVX-208, inhibitor technology, applied in the field of pharmaceutical and chemical industry, can solve the problems of complex pharmacokinetics of drug interactions, and achieve the effect of improving drug safety, mild conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Synthesis of Example 1 Lead Compound RVX-208
[0082] The synthetic route of BRD4 inhibitor RVX-208 provided by the present invention is as follows:
[0083]
[0084] (1) Preparation of intermediate 2 (i.e. compound 2): 3,5-dimethoxybenzoic acid
[0085]
[0086]Add 3,5-dihydroxybenzoic acid (1.54g, 10mmol) into a 100mL single-necked flask, add 20mL of acetone to dissolve it, add potassium carbonate (4.14g, 30mmol) at room temperature, and simultaneously add 3.5mL of dimethyl sulfate dropwise, Heated to 55°C and the reaction was refluxed overnight. After the reaction is complete, concentrate under reduced pressure to recover acetone, add 30mL of water, and then add 30% sodium hydroxide solution to adjust the pH to 14, react at 75°C for 4 hours, and cool the system to room temperature after hydrolysis is complete. The pH was adjusted to about 6 with concentrated hydrochloric acid, and a large amount of white solid precipitated out. After filtering, washing with w...
Embodiment 2R
[0130] The synthesis of embodiment 2RB-X
[0131]
[0132] The synthesis method of the target compound RB-X is similar to that of RVX-208, the difference is that compound 9A is replaced by 9B, the corresponding compound is 10B, and 10B reacts with compound 11 to obtain RB-X. specific,
[0133] (1) Preparation of compound 8
[0134] The synthesis of compound 8 refers to Example 1.
[0135] (2) Preparation of compound 10B
[0136] Preparation of Intermediate 10B: 2-(4-Hydroxy-3,5-dimethoxyphenyl)-5,7-dimethoxyquinazolin-4(3H)-one
[0137]
[0138] Compound 8 (1.96g, 10mmol) was added to a 100mL single-necked flask, and 30mL N,N-dimethylformamide was added to dissolve it, while compound 9B, namely 3,5-dimethoxy-4-hydroxybenzaldehyde (2.00 g, 11mmol), sodium bisulfite (0.95g, 5mmol) and p-toluenesulfonic acid monohydrate (1.25g, 12 mmol), after stirring evenly, the system was heated to 120°C and refluxed for 16 hours. After the reaction, add 20 mL of water to the reacti...
Embodiment 3
[0147] The synthesis of embodiment 3 compound RA-G
[0148] The synthetic route of RA-G provided by the present invention is as follows:
[0149]
[0150] Preparation of target compound RA-G: (5,7-dimethoxy-4-oxo-3,4-dihydroquinazolin-2-yl)-2,6-dimethylphenyl 2-bromo- 2-Methylpropionate
[0151]
[0152] Add compound 13, 2-bromoisobutyric acid (2.00g, 12mmol) into a 100mL single-necked bottle, add 20mL N, N-dimethylformamide to dissolve it, and add EDCI (3.83 g, 20mmol), DMAP (1.22g, 10mmol), after 0.5 hours, compound 10A (3.26g, 10mmol) was added and reacted at room temperature for 16 hours. After the reaction, add 30 mL of water to the system, extract three times with ethyl acetate (3*20 mL), combine the organic phases, dry with anhydrous sodium sulfate, filter, and distill under reduced pressure to obtain the mixture, which is separated and purified by column chromatography (elution The solution was dichloromethane / methanol, the volume ratio was 20:1), and a white ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com