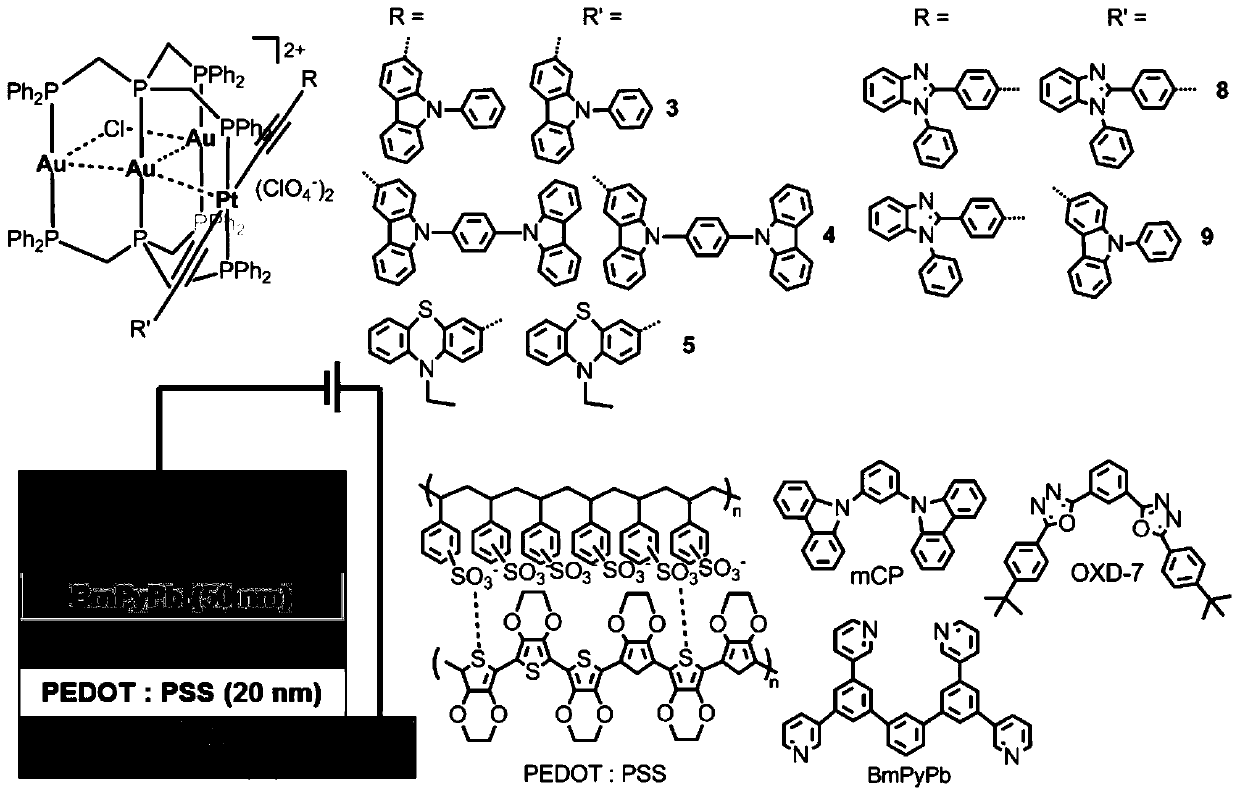
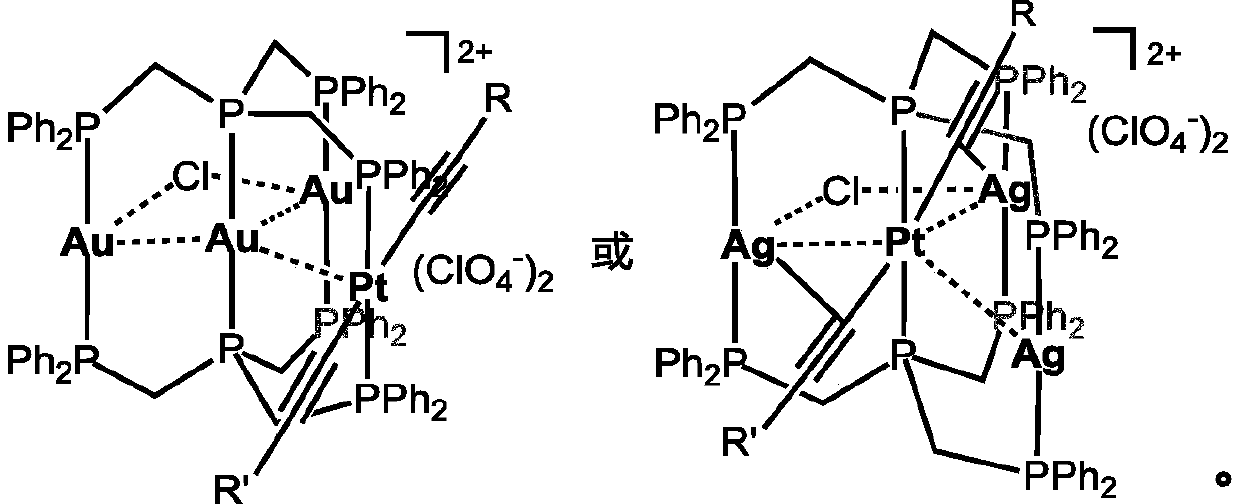
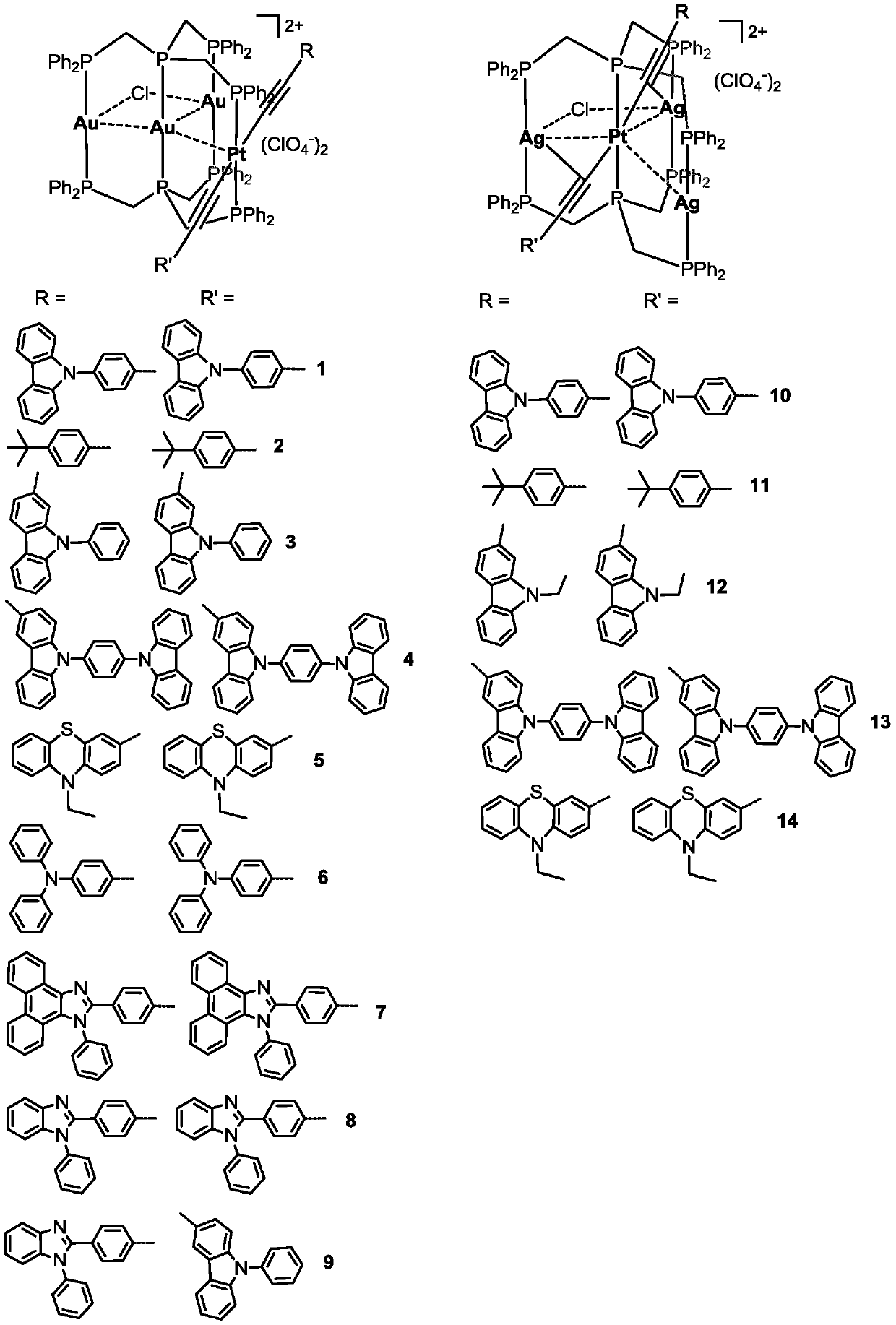
A kind of phosphorescent ptm3 heterotetranuclear complex and its preparation method and use
A complex, phosphorescence technology, applied in chemical instruments and methods, luminescent materials, semiconductor/solid-state device manufacturing, etc., can solve the problems of incomplete chromaticity, high price, shortage of iridium resources, etc., and achieve excellent performance and high conversion efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1: the preparation of complex 1
[0065] Dissolved Pt(PPh 3 ) 2 (C≡C-4-C 6 h 4 Carb-9) 2 (62.6mg, 0.05mmol) in dichloromethane solution was added (Ph 2 PCH 2 ) 3 P(62.8mg, 0.1mmol), Au(tht)Cl(48mg, 0.15mmol), NH 4 ClO 4(18 mg, 0.15 mmol). The reaction solution turned light yellow after being stirred at room temperature for 8 hours. The product was purified by silica gel column chromatography using CH 2 Cl 2 / MeOH (V / V=10:0.5) was used as the eluent to collect the main product. Yield: 68%. 1 H NMR (CDCl 3 ,ppm):8.18(d,4H,J=7.72Hz), 7.93-7.97(m,16H),7.72(d,8H,J=6.88Hz),7.50-7.54(m,16H),7.39-7.42( m, 14H), 7.31-7.34(m, 10H), 7.16(t, 8H, J=7.52Hz), 6.90(d, 4H, J=8.36Hz), 5.70(d, 4H, J=8.32Hz), 4.17(br,4H),3.67(br,8H). 31 P NMR (CDCl 3 ,ppm):30.4(t, 4P,J P-P =31.5Hz), 22.7(m,2P,J P-P =25.0Hz), 4.2(t,2P,J P-P =25.1Hz,J Pt-P = 2676Hz).HRMS(ESI): According to C 118 h 96 Au 3 ClN 2 P 8 Pt[M-2ClO 4 ] 2+ Calculated value: 1305.1880; Measured...
Embodiment 2
[0066] Embodiment 2: the preparation of complex 2
[0067] Preparation method is identical with method in embodiment 1, only is to use Pt(PPh 3 ) 2 (C≡C-C 6 h 4 Bu t -4) 2 Instead of Pt(PPh 3 ) 2 (C≡C-4-C 6 h 4 Carb-9) 2 , Yield: 72%. 1 H NMR (CDCl 3 ,ppm): 7.89-7.93(m,8H),7.74-7.77(m,8H,7.66-7.70(m,8H),7.49-7.52(m,14H), 7.31-7.34(m,14H),7.02- 7.05(m,8H,6.54(d,4H,J=8.04Hz),5.41(d,4H,J=8.04Hz),4.08(br,4H),3.85(br,4H),3.54(br,4H) 0.98-1.2(m,18H). 31 P NMR (CDCl 3 ,ppm):29.1(t,4P,J P-P =32.6Hz), 17.8(m,2P,J P-P =30.0Hz),4.8(t,2P,J P-P =27.1Hz,J Pt-P =2694Hz).HRMS(ESI):according to C 102 h 98 Au 3 ClN 2 P 8 Pt[M-2ClO 4 ] 2+ Calculated value: 1196.1927; Measured value: 1196.1976.IR(KBr,cm -1 ):2105w(C≡C), 1100s(ClO 4 - ).
Embodiment 3
[0068] Embodiment 3: the preparation of complex 3
[0069] Preparation method is identical with method in embodiment 1, only is to use Pt(PPh 3 ) 2 (C≡C-2-PhCarb-9) 2 Instead of Pt(PPh 3 ) 2 (C≡C-4-C 6 h 4 Carb-9) 2 . Yield: 70%. 1 H NMR (CDCl 3 ,ppm): 8.01-8.03(m,8H),7.74-7.77(m,10H),7.66-7.69(m,10H),7.51-7.55(m,12H), 7.43-7.49(m,12H),7.37 -7.41(m,16H),6.95(m,10H),6.78(d,2H,J=8.4Hz), 6.02(s,2H),5.78(d,2H,J=8.4Hz),4.13(br, 4H), 4.03(br,4H), 3.65(br,4H). 31 P NMR (CDCl 3 ,ppm):29.6(t,4P,J P-P =32.0Hz), 18.1(m,2P,J P-P =30.6Hz), 6.2(t,2P,J P-P =30.0Hz,J Pt-P =2726Hz).HRMS(ESI):according to C 118 h 96 Au 3 ClN 2 P 8 Pt[M-2ClO 4 ] 2+ Calculated value: 1305.1880; Measured value: 1305.1909.IR (KBr, cm -1 ):2099w(C≡C),1100s(ClO 4 - ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com