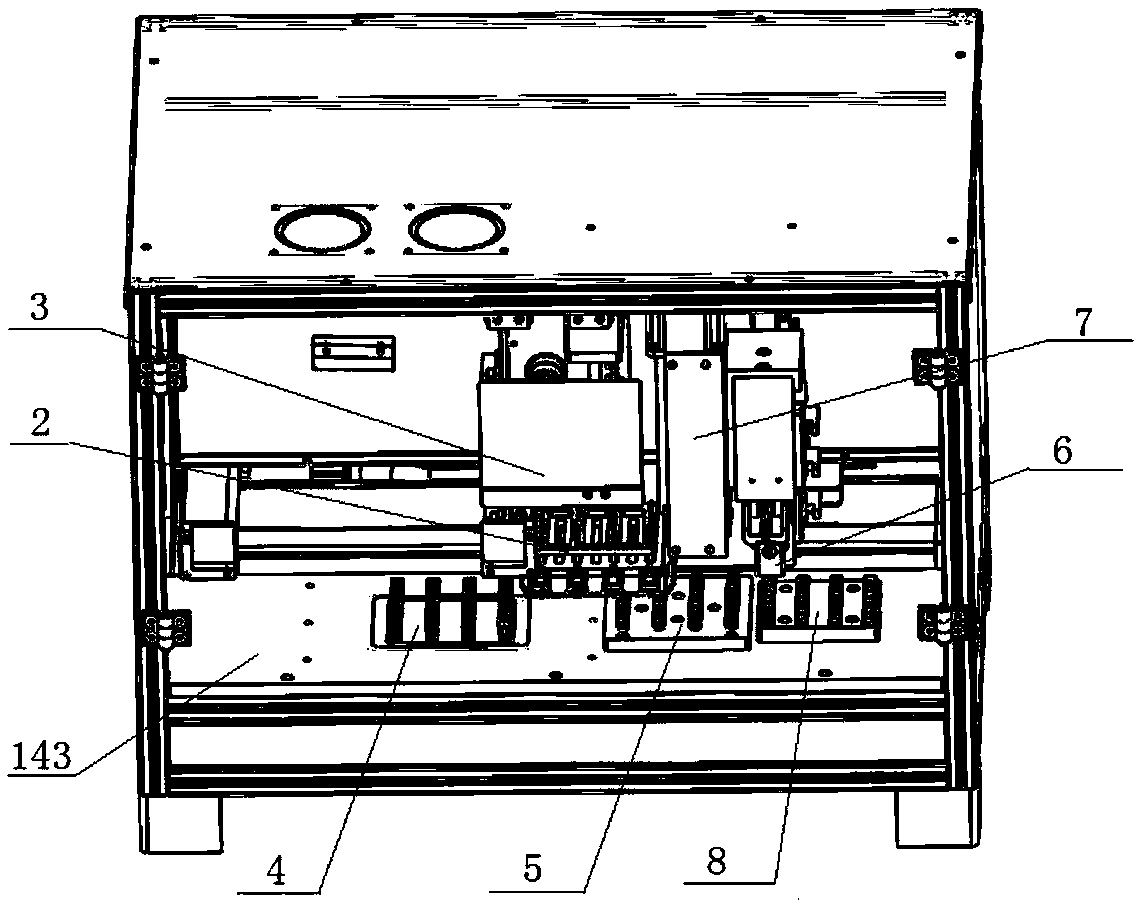
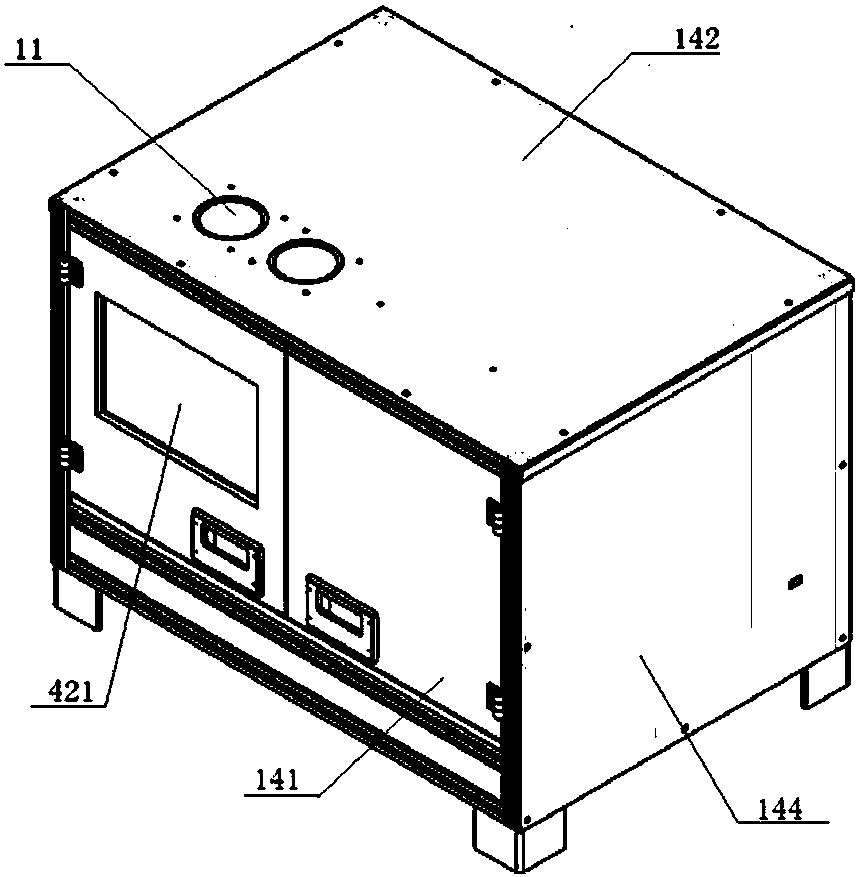
Nucleic acid extraction method
An extraction method and nucleic acid technology, applied in DNA preparation, recombinant DNA technology, etc., can solve the problems of manual transfer of nucleic acid eluent, many washing and elution steps, and intensified fragmentation of nucleic acid, so as to avoid human interference and pollution, nucleic acid The effect of simplifying the extraction process, improving accuracy and consistency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0161] Minimum Detection Limit Testing for DNA References
[0162] Use the nucleic acid extraction method of the present invention to manually operate the test, and use a nucleic acid extractor to automatically extract nucleic acid for testing to verify the feasibility:
[0163] Using the nucleic acid extraction method of the present invention to manually operate the experiment, the steps are as follows:
[0164] Step 1. Obtain a PCR reaction tube containing lysis solution and magnetic beads (8-row PCR tubes are used in this experiment, and the sequence of holes is A, B, C, D, E, F, G, H). Add samples to the first PCR reaction tube; add 10 2 CFU / ml, Bacillus pertussis (DNA reference), D4, E4, F4 wells were added with 10 3 CFU / ml, Bacillus pertussis (DNA reference); G4, H4 wells were added with 10 4 CFU / ml, Bacillus pertussis (DNA reference); add 10 to the A5 well of the second PCR reaction tube 4 CFU / ml, Bacillus pertussis (DNA reference), B5, C5, D5 wells were added with ...
Embodiment 2
[0181] Anti-pollution test of nucleic acid extractor
[0182] 2.1 The first set of anti-contamination tests for DNA reference
[0183] Using the nucleic acid extractor of the present invention to operate the experiment, the steps are as follows:
[0184] S1. Obtain a PCR reaction tube containing lysis solution and magnetic beads (8-row PCR tubes are used in this experiment, and the sequence of holes is A, B, C, D, E, F, G, H). Add samples, the samples are arranged as Figure 15 shown,
[0185] The A3 well position of the first PCR reaction tube is a DNA strong positive reference 10 6 CFU / m (Bacillus pertussis), B3 hole is a negative sample, C3 hole is a DNA strong positive reference 10 6 CFU / m (Bacillus pertussis), D3 hole is a negative sample, E3 hole is a DNA strong positive reference 10 6 CFU / m (Bacillus pertussis), F3 well is a negative sample, G3 well is a DNA strong positive reference 10 6 CFU / m (Bacillus pertussis), the H3 hole is a negative sample;
[0186] The ...
Embodiment 3
[0239] 3.1. Precision testing of DNA reference materials
[0240] Using the nucleic acid extractor of the present invention to operate the experiment, the steps are as follows:
[0241] S1. Obtain a PCR reaction tube containing lysis solution and magnetic beads (8-row PCR tubes are used in this experiment, and the sequence of holes is A, B, C, D, E, F, G, H). Add samples, the samples are arranged as Figure 15 As shown, prepare four 8-row PCR reaction tubes containing lysis buffer and magnetic beads, and add DNA reference material (10 6 CFU / ml, Bacillus pertussis);
[0242] Prepare 4 8-row PCR reaction tubes containing the pretreatment solution and place them in the tube rack in the pretreatment area;
[0243] S2. Put the four PCR reaction tubes after loading into the heating load of the lysis area, and put each hole in a sleeve of the heating load; liquid heating, the temperature rises, the exhaust fan is turned on, and after the heating is completed, the pyrolysis liquid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com