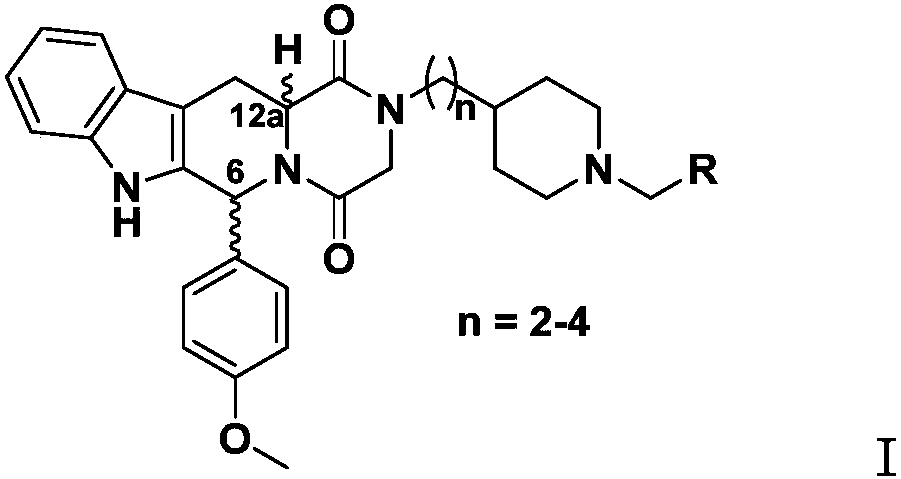
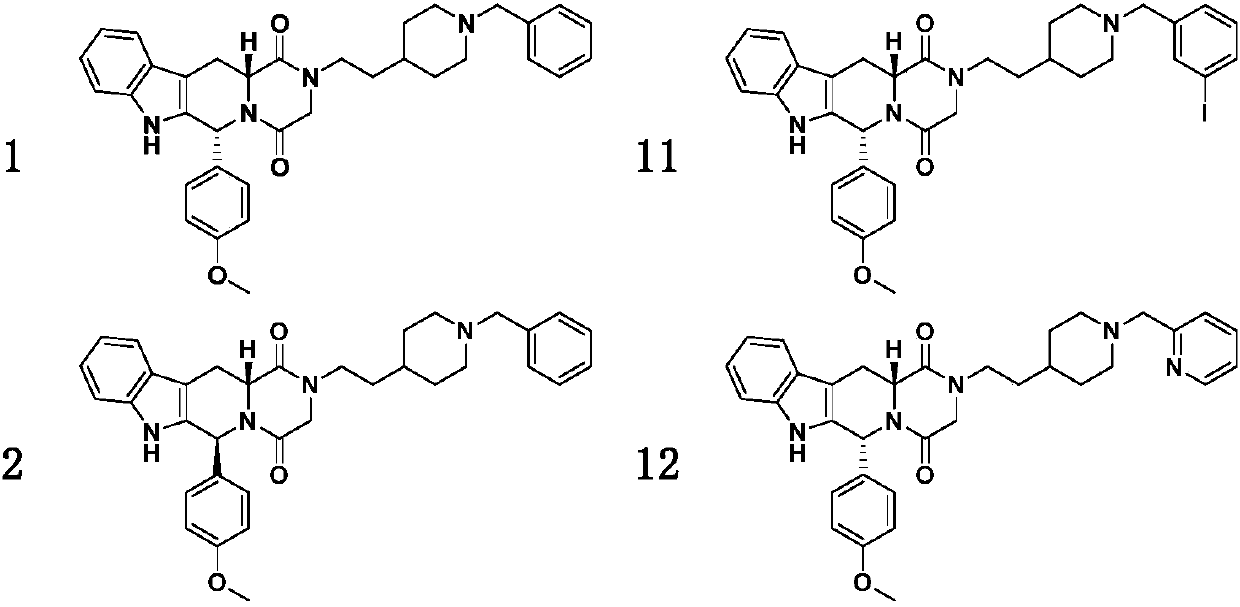
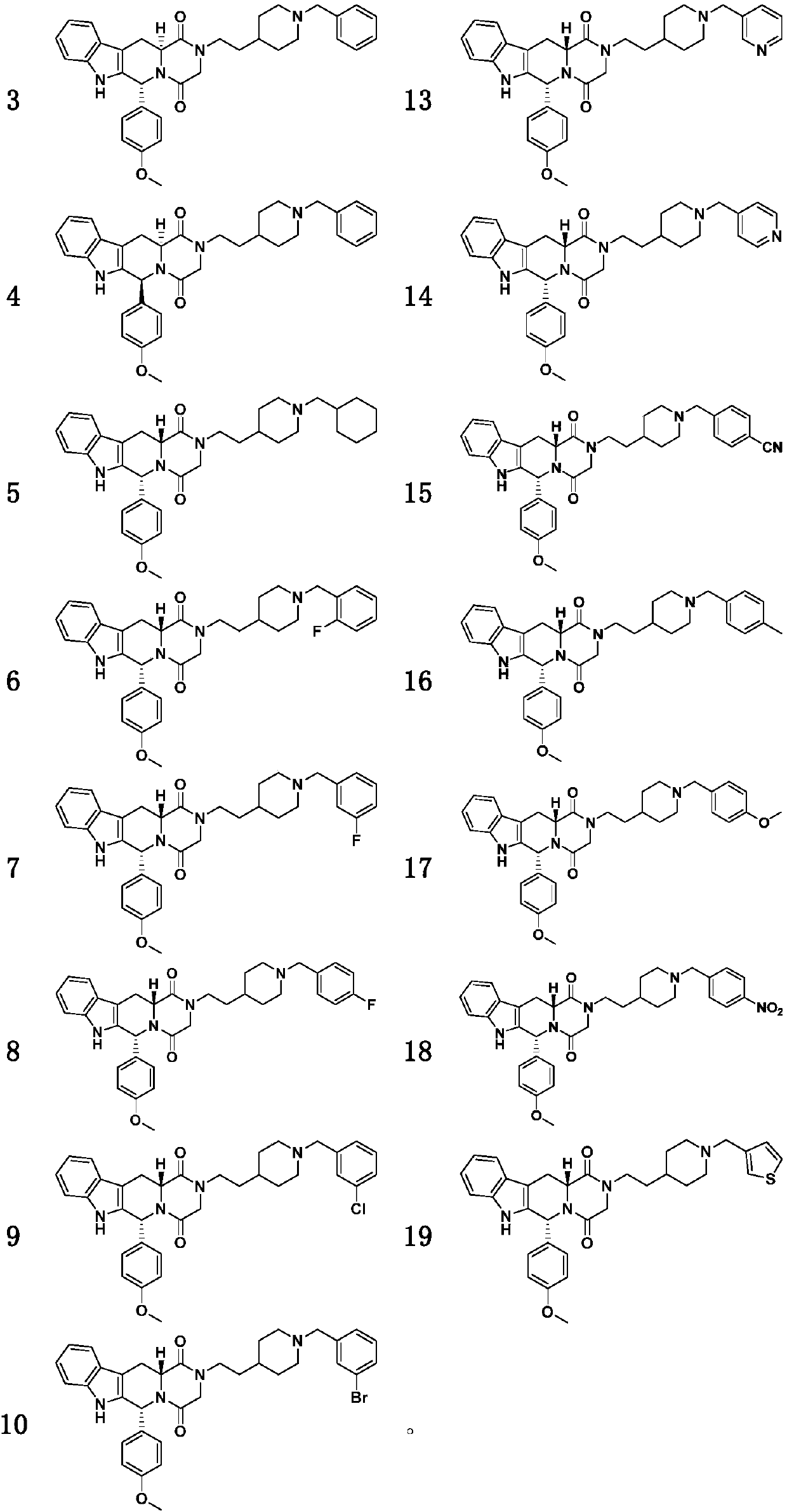
4-methoxyphenyl substituted tetrahydro-beta-carboline piperazine dione derivative and uses thereof
An unsubstituted, alkoxy-based technology, applied in the fields of medicinal chemistry and pharmacotherapeutics, can solve the problems of terminating the disease process and single target
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The preparation method of the compound of the present invention is not limited to the specific preparation method described in the present invention, and can optionally be combined with various synthetic methods described in this description or known in the art to make it conveniently. Such a combination can be obtained by It is easy for those skilled in the art to which the present invention pertains.
[0056] Usually, in the preparation process, each reaction is usually carried out in an inert solvent at room temperature to reflux temperature (such as 0°C to 80°C, preferably 0°C to 50°C). The reaction time is usually 0.1-60 hours, preferably 0.5-48 hours.
[0057] The preparation method of the compound of the present invention may comprise the following steps:
[0058]
[0059] (1) In an inert solvent, carry out Pictet-Spengler reaction with 4-methoxybenzaldehyde and D-tryptophan methyl ester hydrochloride or L-tryptophan methyl ester hydrochloride, thereby formin...
Embodiment 1
[0096] Example 1 (1R,3R)-1-(4-methoxyphenyl)-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid methyl ester (intermediate II-1) preparation of
[0097]
[0098] Add 817mg (6mmol) of 4-methoxybenzaldehyde, 1.528g (6mmol) of D-tryptophan methyl ester hydrochloride, and 20mL of isopropanol into a round-bottomed flask, and heat to reflux for 24 hours. After the reaction, spin off As a solvent, add an appropriate amount of saturated sodium bicarbonate solution to adjust the pH to 7-8, extract with ethyl acetate, wash with saturated brine, dry over anhydrous sodium sulfate, filter, evaporate the solvent under reduced pressure, and the residue is separated and purified by silica gel column chromatography. Eluted with ethyl acetate / petroleum ether=1 / 3, 876 mg of yellow solid was obtained with a yield of 43%.
[0099] 1 H NMR (400MHz, CDCl 3 )δ7.53(d, J=7.6Hz, 1H), 7.47(s, 1H), 7.29(d, J=8.5Hz, 2H), 7.21(d, J=6.9Hz, 1H), 7.18–7.08( m,2H),6.89(d,J=8.6Hz,2H),5.20(s,1H),3.97(dd,J=11...
Embodiment 2
[0100] Example 2 (1S,3R)-1-(4-methoxyphenyl)-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid methyl ester (intermediate II-2) preparation of
[0101]
[0102] The specific implementation steps are the same as in Example 1, the solvent isopropanol is replaced by ethanol, the silica gel column chromatography is separated and purified, the eluent is ethyl acetate / petroleum ether=1 / 3-1 / 1, and 667 mg of white solid is obtained, and the yield is 33 %.
[0103] 1 H NMR (400MHz, CDCl 3 )δ7.55(d,J=6.6Hz,2H),7.17(ddd,J=22.0,13.9,6.0Hz,5H),6.86(d,J=8.5Hz,2H),5.41(s,1H), 3.99(t, J=6.0Hz, 1H), 3.79(s, 3H), 3.72(s, 3H), 3.28(dd, J=15.3, 5.3Hz, 1H), 3.15(dd, J=15.5, 6.5Hz ,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com