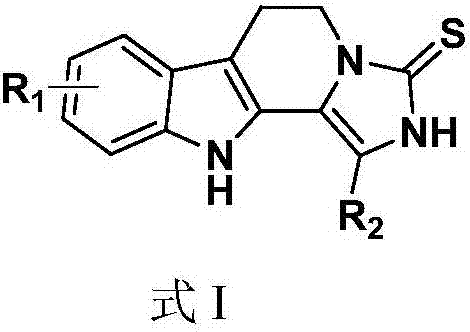
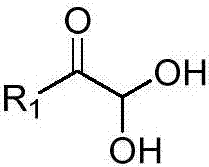
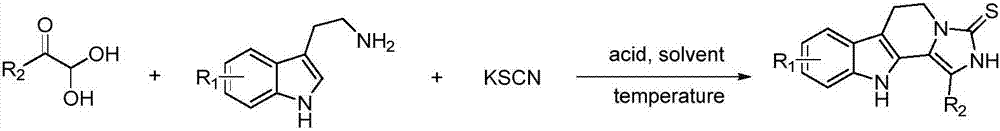
1,2,3,4-tetrahydro-beta-carboline-N-heterothioiminazole compound as well as preparation and application
A technology of heterothiimidazole and compound, which is applied in the field of pharmacy and achieves the effects of convenient post-processing, wide application range and wide substrate range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 1-Phenyl-2,5,6,11-tetrahydro-3H-imidazo[1',5':1,2]pyrido[3,4-b]indole-3-thione
[0050]
[0051]Tryptamine (1.0mmol, 160.2mg), 2,2-dihydroxy-1-phenylethanone (1.2mmol, 182.4mg), potassium thiocyanate (2.0mmol, 194mg), trifluoroacetic acid (1.0mmol, 76 μL) was added to a high-pressure and high-temperature-resistant glass sealed tube, and then added to 5 mL of anhydrous acetonitrile. After the addition, the reaction was carried out in a 40-degree oil bath. The reaction was detected by TLC, and the reaction was completed after 8 hours. After the reaction was completed, the obtained reaction solution was concentrated, extracted with water and ethyl acetate, and the obtained organic phase was dried and concentrated to obtain a residue, which was recrystallized with ethanol to obtain a yellow solid with a yield of 80%.
[0052] Yellow solid, m.p.>250℃, 1 H NMR(500MHz,DMSO)δ12.70(s,1H),10.66(s,1H),7.70-7.66(m,2H),7.55-7.51(m,3H),7.44(t,J=7.5Hz, 1H), 7.39(d, J=8....
Embodiment 2
[0053] The yield situation comparison of this reaction of embodiment 2 under different reaction conditions
[0054]
[0055] Table 1
[0056] sequence
Embodiment 3
[0057] Example 3 1-(4-(trifluoromethyl)phenyl)-2,5,6,11-tetrahydro-3H-imidazo[1',5':1,2]pyrido[3,4 -b] indole-3-thione
[0058]
[0059] The synthesis steps are the same as in Example 1, except that 2,2-dihydroxy-1-phenylethanone is replaced by 2,2-dihydroxy-1-(p-trifluoromethylphenyl)ethanone to obtain a yellow solid, which is recovered as The rate is 77%.
[0060] Yellow solid, m.p.>250℃, 1 H NMR (500MHz, DMSO) δ12.86(s, 1H), 10.80(s, 1H), 7.86(d, J=8.0Hz, 4H), 7.55(d, J=7.9Hz, 1H), 7.39(d ,J=8.1Hz,1H),7.14(dd,J=11.1,3.9Hz,1H),7.06(t,J=7.4Hz,1H),4.15(t,J=6.7Hz,2H),3.15(t ,J=6.7Hz,2H). 13 C NMR (125MHz, DMSO) δ162.4, 155.4, 138.0, 132.6, 128.6 (q, J = 31.6Hz), 128.0, 126.5 (q, J = 3.5Hz), 126.0, 124.7 (d, J = 270Hz), 124.2, 123.1, 120.7, 120.0, 119.4, 118.7, 112.7, 111.1, 42.0, 20.0. HRMS (ESI): m / z calcd for (C 20 h 14 f 3 N 3 S+H) + :386.0933; found: 386.0934.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com