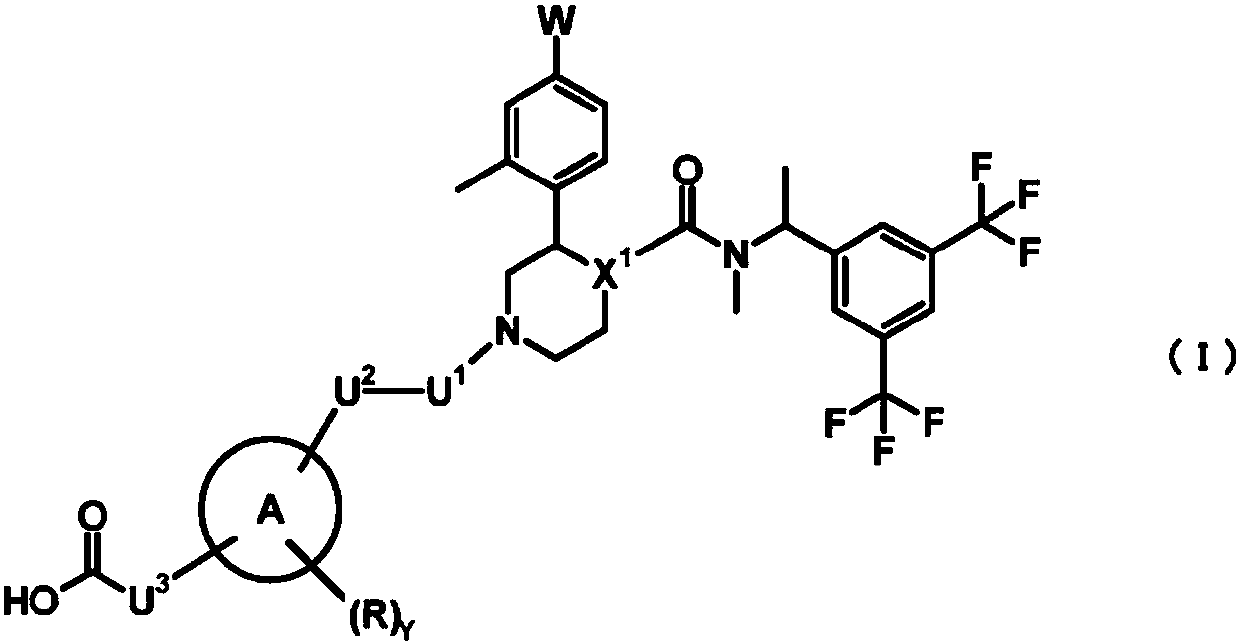
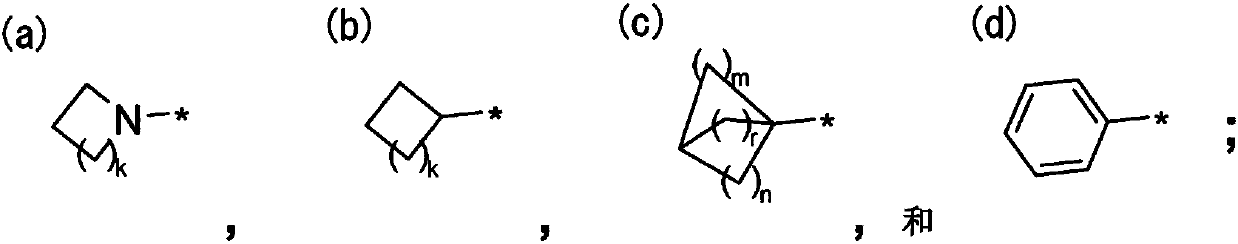
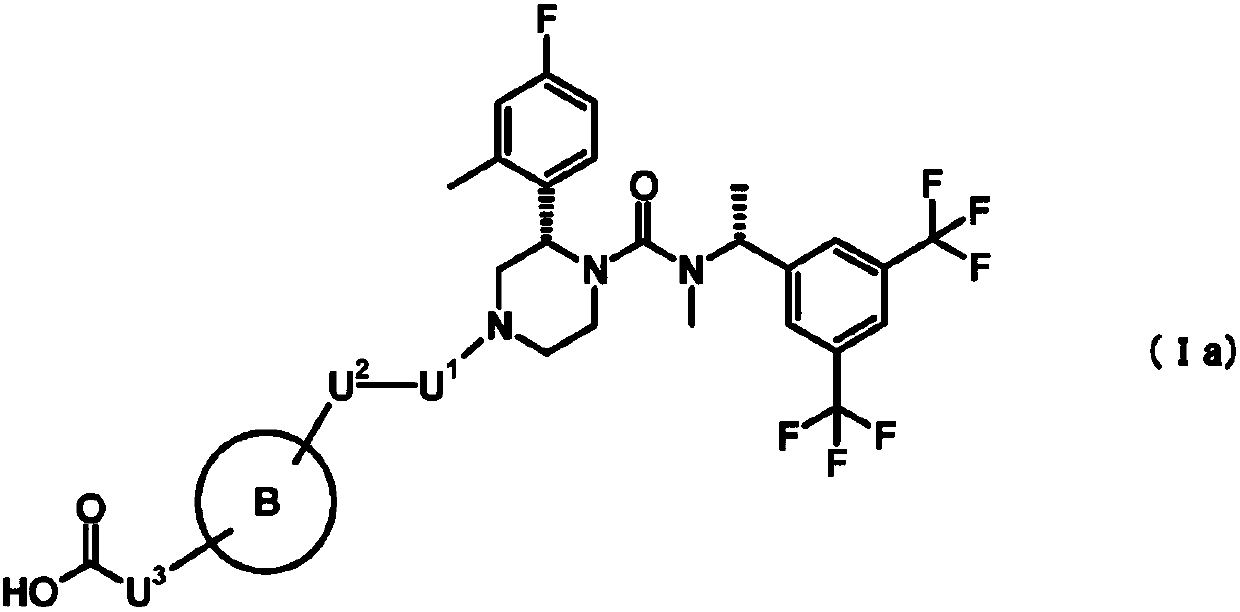
Nk1 receptor antagonist
A compound and pharmaceutical technology, applied in the field of NK1 receptor antagonists, can solve the problem that NK1 receptor antagonists do not have any description or prompt, and achieve excellent NK1 receptor antagonist activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0212] The present invention will be further explained in more detail by the following reference examples, examples and test examples. However, the present invention is not limited thereto.
reference example 1
[0214] {4-[(S)-4-{[(R)-1-(3,5-bistrifluoromethylphenyl)ethyl]methylcarbamoyl}-3-(4-fluoro-2- Methylphenyl)piperazin-1-yl]cyclohexyl}acetate
[0215] (S)-2-(4-fluoro-2-methylphenyl)piperazine-1-carboxylic acid [(R)-1-(3,5-bistrifluoromethylphenyl)ethyl]methyl A solution of amide mesylate (1:1) (0.059g), methyl 4-oxo-cyclohexyl)acetate (0.026g) and triethylamine (0.020g) in tetrahydrofuran (3mL) was stirred at room temperature 10 minutes. To the reaction mixture were added sodium triacetoxyborohydride (0.042 g) and acetic acid (0.012 g) at room temperature, and the mixture was stirred at the same temperature overnight. To the reaction mixture was added saturated aqueous sodium bicarbonate solution, and the resulting mixture was extracted with ethyl acetate. The organic layer was washed with brine and dried over anhydrous magnesium sulfate, and the solvent was removed under reduced pressure. The obtained crude product was purified by silica gel column chromatography (eluent: ...
reference example 2 and 3
[0217] cis-benzyl 4-ethoxycarbonylmethylcyclohexanecarboxylate (reference example 2) and trans-benzyl 4-ethoxycarbonylmethylcyclohexanecarboxylate (reference example 3)
[0218] To a solution of 4-ethoxycarbonylmethylcyclohexanecarboxylic acid (8.96 g) in dichloromethane (50 mL) was added N,N-dimethylformamide (0.010 mL), and the resulting Oxalyl chloride (10.62 g) was added dropwise to the mixture, and the mixture was stirred at the same temperature for 2 hr. The reaction mixture was concentrated under reduced pressure, dichloromethane was added to the residue, and concentrated again. This step was repeated 3 times. To the obtained residue were added dichloromethane (50 mL) and triethylamine (6.35 g), to the resulting mixture was added dropwise benzyl alcohol (5.43 g) under ice-cooling, and the mixture was stirred at room temperature overnight. To the reaction mixture was added saturated aqueous sodium bicarbonate solution, and the resulting mixture was extracted with ethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com