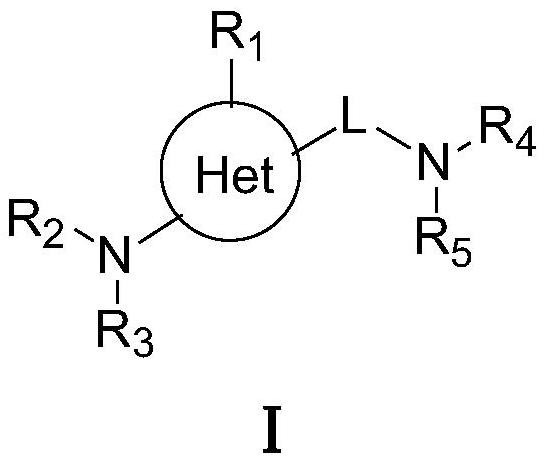
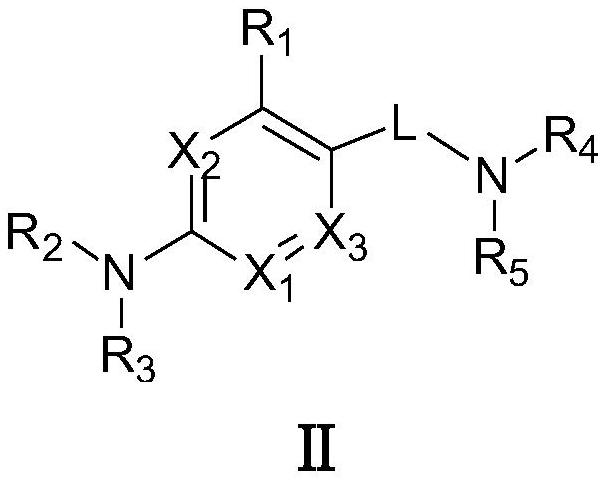
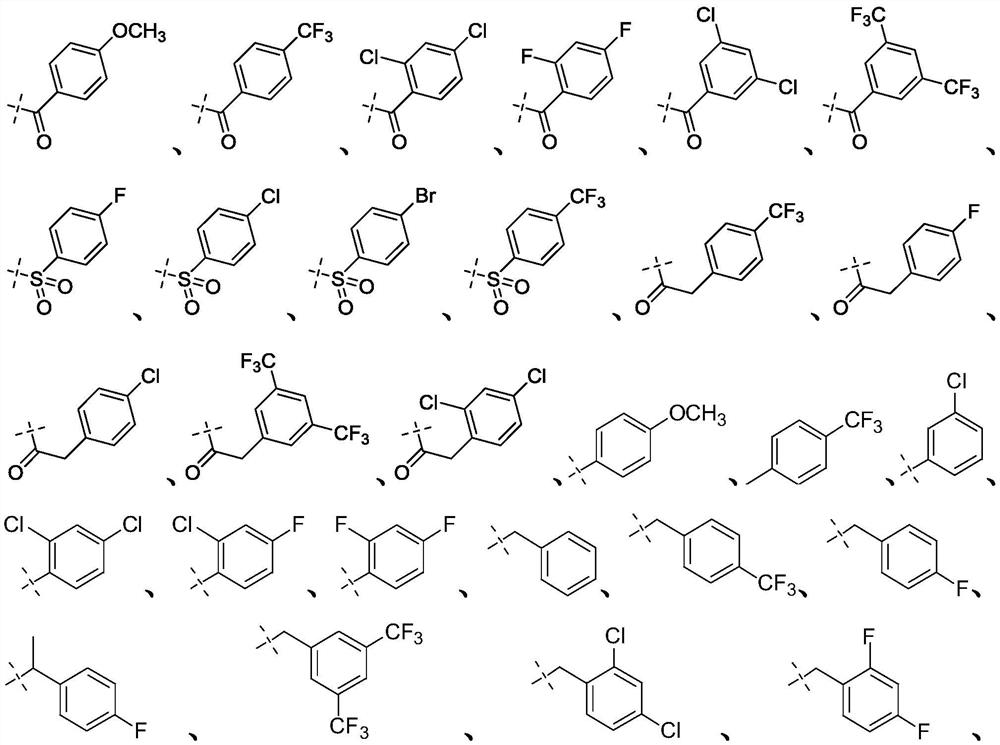
Targeted nk1 receptor antagonists and their application in the treatment of chemotherapy-induced nausea and vomiting
A compound and chemical bond technology, applied in the field of adjuvant chemotherapy drugs for tumors, can solve problems such as large side effects and lack of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] The synthesis of embodiment 1 compound A1-A18
[0109] Compounds A1-A18 can be synthesized using the following reaction formula:
[0110]
[0111] Reagents and conditions: (i-A) a. Thionyl chloride, reflux for 3 hours; b. Dichloromethane, DIEA (N,N-diisopropylethylamine); (ii-A) o-tolylmagnesium chloride (O- tolyl magnesium chloride), tetrahydrofuran, dichlorodicyanobenzoquinone or manganese acetate; (iii-A) isopropanol, nitrogen methylpiperazine; (iv-A) methanesulfonic acid; (v-A) N-bromobutyl Diimide, sodium methoxide, methanol; (vi-A) red aluminum, toluene; (vii-A) N,N-diisopropylethylamine, dichloromethane;
[0112] Preparation of Intermediate A-1
[0113] The raw material 5-carboxy-2-chloropyridine (1eq) was dissolved in 10ml of thionyl chloride, and refluxed for 2h. After cooling to room temperature, the solvent was spin-dried and dissolved with an appropriate amount of dry dichloromethane to obtain a yellow solution for use. Then tert-butylamine (1.1eq) an...
Embodiment 2
[0155] Embodiment 2 compound B1-B15 is synthesized
[0156]
[0157] Reaction reagents and conditions: (i-B) concentrated sulfuric acid and methanol (ii-B) isopropanol, nitrogen methyl piperazine; (iii-B) lithium hydroxide and water; (iv-B) 2-(7-azo Benzotriazole)-tetramethyluronium hexafluorophosphate, N,N-diisopropylethylamine, amine derivatives;
[0158] Intermediate B-8 Synthesis
[0159] Dissolve 1 mmol of intermediate B-7 in 15 ml of methanol, add two drops of concentrated sulfuric acid, react at room temperature for 10 h, concentrate and dissolve in ethyl acetate, wash with saturated sodium bicarbonate, water, saturated brine, and anhydrous sodium sulfate Dry and concentrate to obtain intermediate B-8, which is used directly without purification. 1 H-NMR (DMSO-d 6 ,400MHz),δ(ppm):8.88(1H,s),7.53(3H,m),7.39(1H,m),7.23(1H,m),3.84(3H,s),2.61(3H,s) .
[0160] Intermediate B-9 Synthesis
[0161] Intermediate 8 (1 mmol) was dissolved in 10 ml of isopropanol, and nitr...
Embodiment 3
[0181] Example 3 Synthesis of Compound C1-C9
[0182] Compound C-1 was prepared by referring to the method of Example 2. Compound C-1 was reacted with different amine derivatives to obtain the target compound. The reaction scheme is as follows:
[0183]
[0184] Reagents and conditions: 2-(7-azobenzotriazole)-tetramethyluronium hexafluorophosphate, N,N-diisopropylethylamine, amine derivatives.
[0185] Take the above-mentioned intermediate C-1 (1mmol) and dissolve it in 10ml DMF (N,N-dimethylformamide), add DIEA (N,N-diisopropylethylamine) (2mmol), and derivatize with different amines (1 mmol) and HBTU (2-(7-azobenzotriazole)-tetramethyluronium hexafluorophosphate) (1 mmol) were reacted overnight at room temperature. The reaction solution was poured into water, extracted with ethyl acetate, and the organic layers were combined, washed 3 times with water, washed with saturated brine, and dried over anhydrous sodium sulfate. After concentration, it was purified by silica ge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com