Construction method of hplc characteristic map of a kind of Chinese patent medicine "Qingyi Lidan Granule"
A technology for clearing the pancreas, promoting gallbladder, and characteristic spectrum, which is applied in the field of construction of HPLC characteristic spectrum of Chinese patent medicines, can solve problems such as difficult product quality, and achieve the effects of easy identification, high similarity and high precision.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
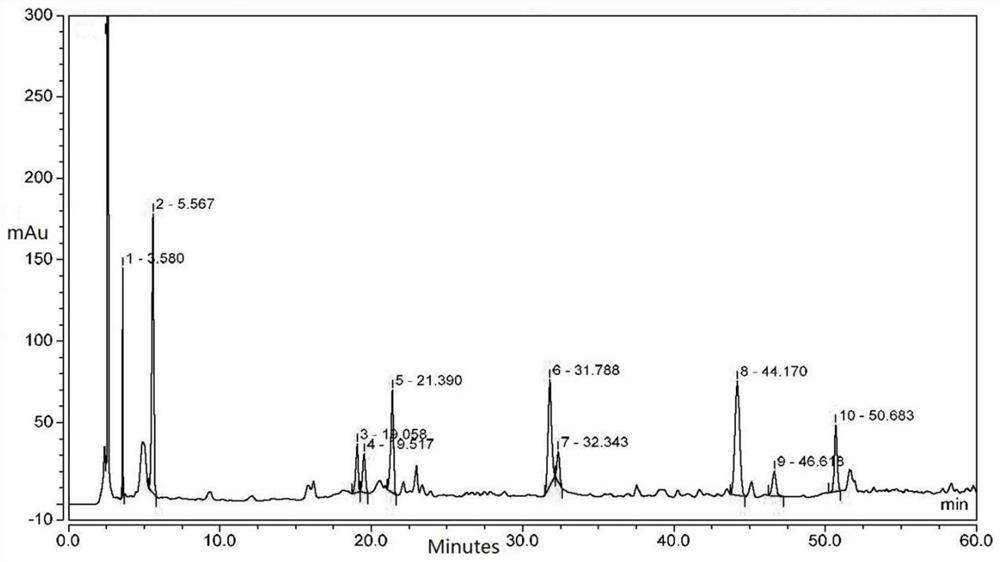
[0029] Embodiment 1: A kind of HPLC characteristic chromatogram construction method of Qingyilidan granules
[0030] Instrument: Agilent 1200 high performance liquid chromatograph, MS205DU analytical balance
[0031] Reagents: Paeoniflorin reference substance, chlorogenic acid reference substance, acetonitrile for liquid chromatographic analysis were chromatographically pure, the rest of the reagents were analytically pure, water was ultrapure water, and Qingyilidan granules were provided by Fusong County Traditional Chinese Medicine Co., Ltd. .
[0032] Preparation of the test solution: take 0.5g of Qingyilidan granule fine powder, accurately weigh it, place it in a stoppered conical flask, accurately add 20ml of methanol, weigh it, ultrasonically treat it for 20 minutes, let it cool, and weigh it again. Determine the weight, use methanol to make up the lost weight, shake well, filter, and take the continuous filtrate.
[0033] Preparation of reference substance solution: r...
Embodiment 2
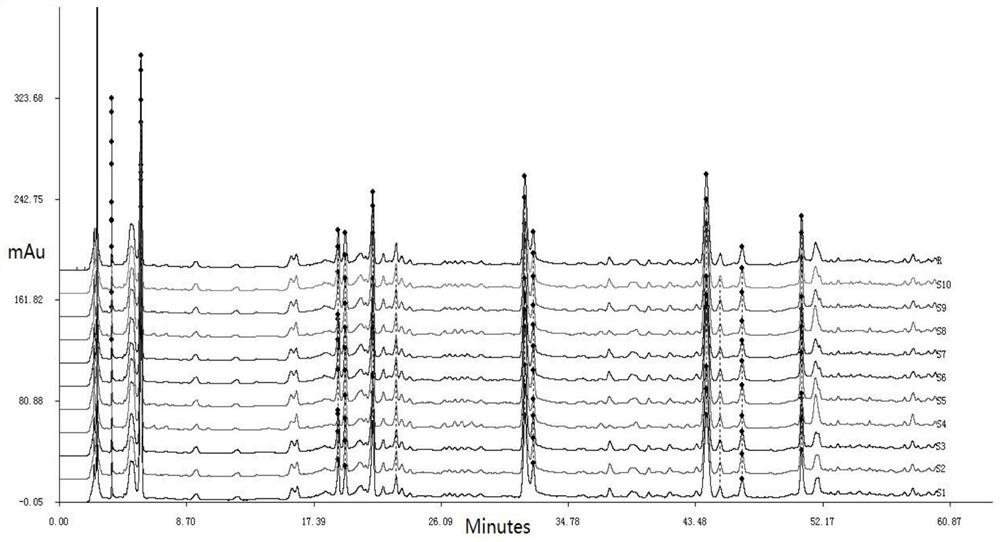
[0038] Embodiment 2: A kind of HPLC characteristic chromatogram construction method of Qingyilidan granules
[0039] Instrument: Agilent 1200 high performance liquid chromatograph, MS205DU analytical balance
[0040] Reagents: Paeoniflorin reference substance, chlorogenic acid reference substance, acetonitrile for liquid chromatographic analysis were chromatographically pure, the rest of the reagents were analytically pure, water was ultrapure water, and Qingyilidan granules were provided by Fusong County Traditional Chinese Medicine Co., Ltd. .
[0041] Preparation of the test solution: take 1.5g of Qingyilidan granule fine powder, accurately weigh it, place it in a conical flask with stopper, accurately add 30ml of methanol, weigh it, ultrasonically treat it for 40 minutes, let it cool, and weigh again. Determine the weight, use methanol to make up the lost weight, shake well, filter, and take the continuous filtrate.
[0042] Preparation of reference substance solution: r...
Embodiment 3
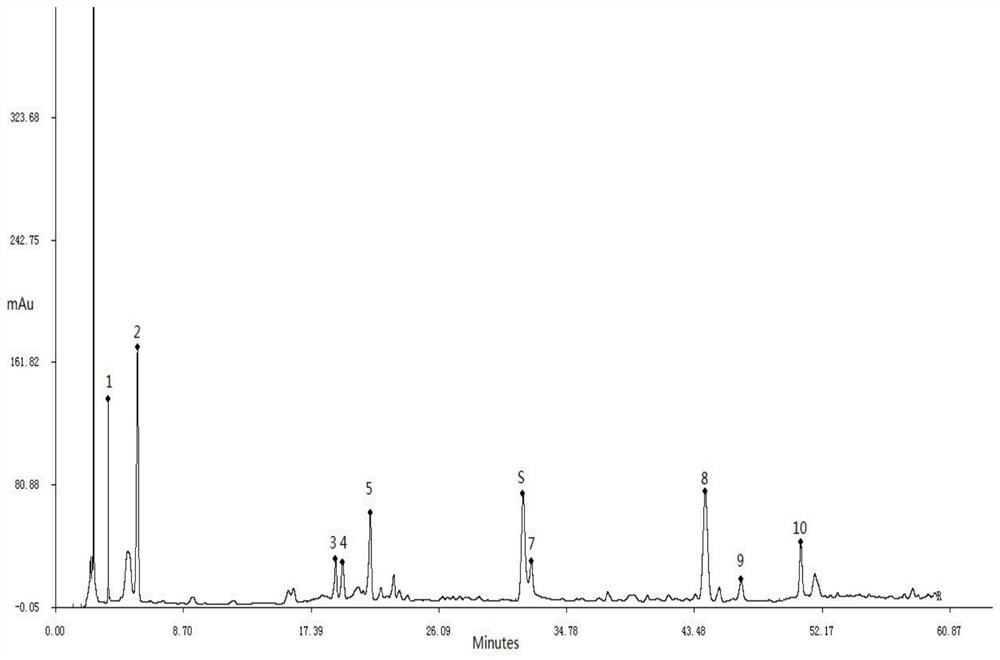
[0048] Embodiment 3: a kind of HPLC characteristic chromatogram construction method of Qingyilidan granules
[0049] Instrument: Dionex U-3000 high performance liquid chromatograph, MS205DU analytical balance
[0050] Reagents: Paeoniflorin reference substance, chlorogenic acid reference substance, acetonitrile for liquid chromatographic analysis were chromatographically pure, the rest of the reagents were analytically pure, water was ultrapure water, and Qingyilidan granules were provided by Fusong County Traditional Chinese Medicine Co., Ltd. .
[0051] Preparation of the test solution: take 1.0 g of Qingyilidan granule fine powder, accurately weigh it, place it in a conical flask with a stopper, add 25 ml of methanol precisely, weigh it, ultrasonically treat it for 30 minutes, let it cool, and weigh it again. Determine the weight, use methanol to make up the lost weight, shake well, filter, and take the continuous filtrate.
[0052] Preparation of reference substance solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com