High-sensitivity chemiluminescence immunoassay kit, and preparation method and application thereof
A chemiluminescence immunoassay and analytical reagent technology, which is applied in the field of high-sensitivity chemiluminescence immunoassay kits and its preparation, can solve the problems of low sensitivity and achieve the effects of improved sensitivity, good repeatability and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
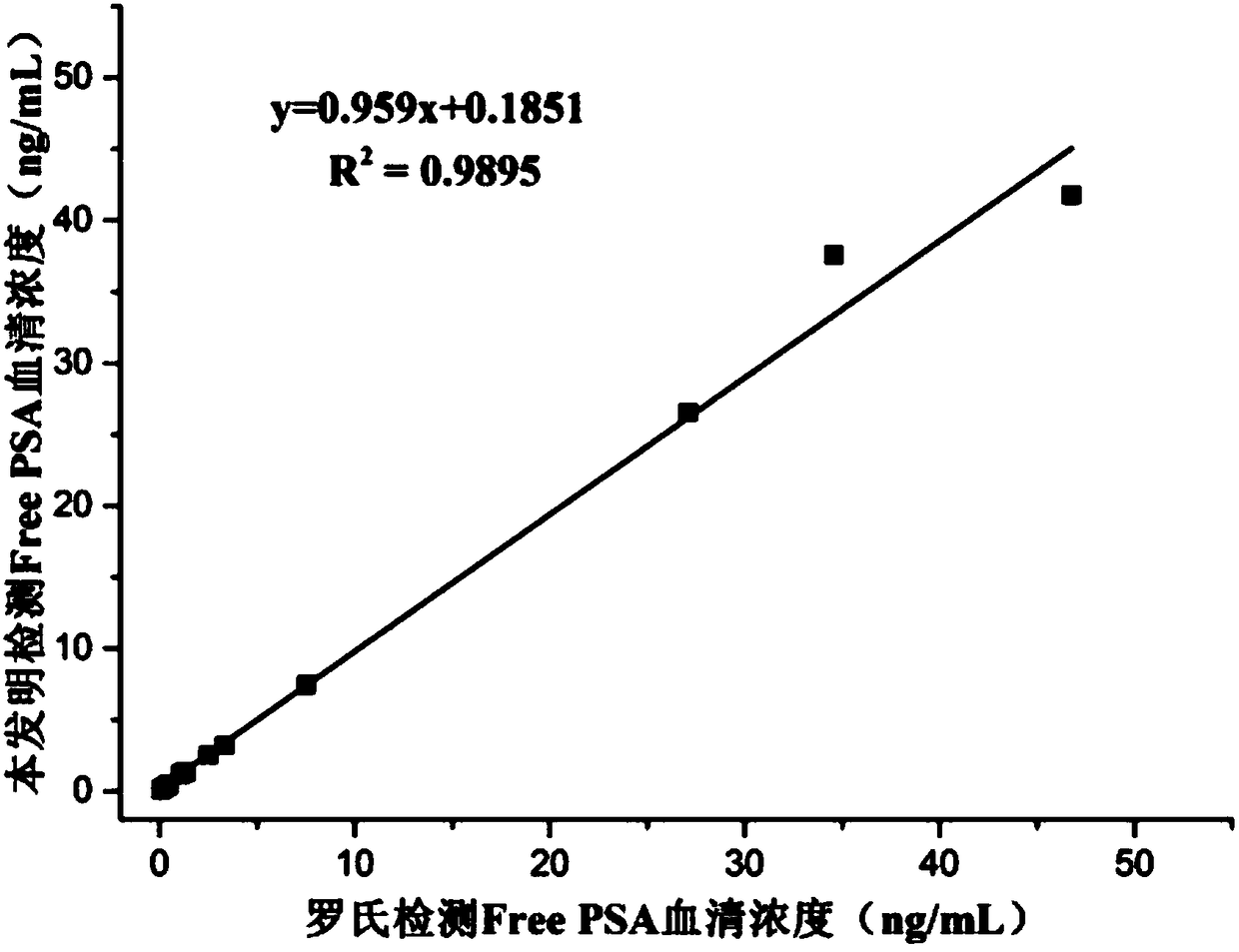
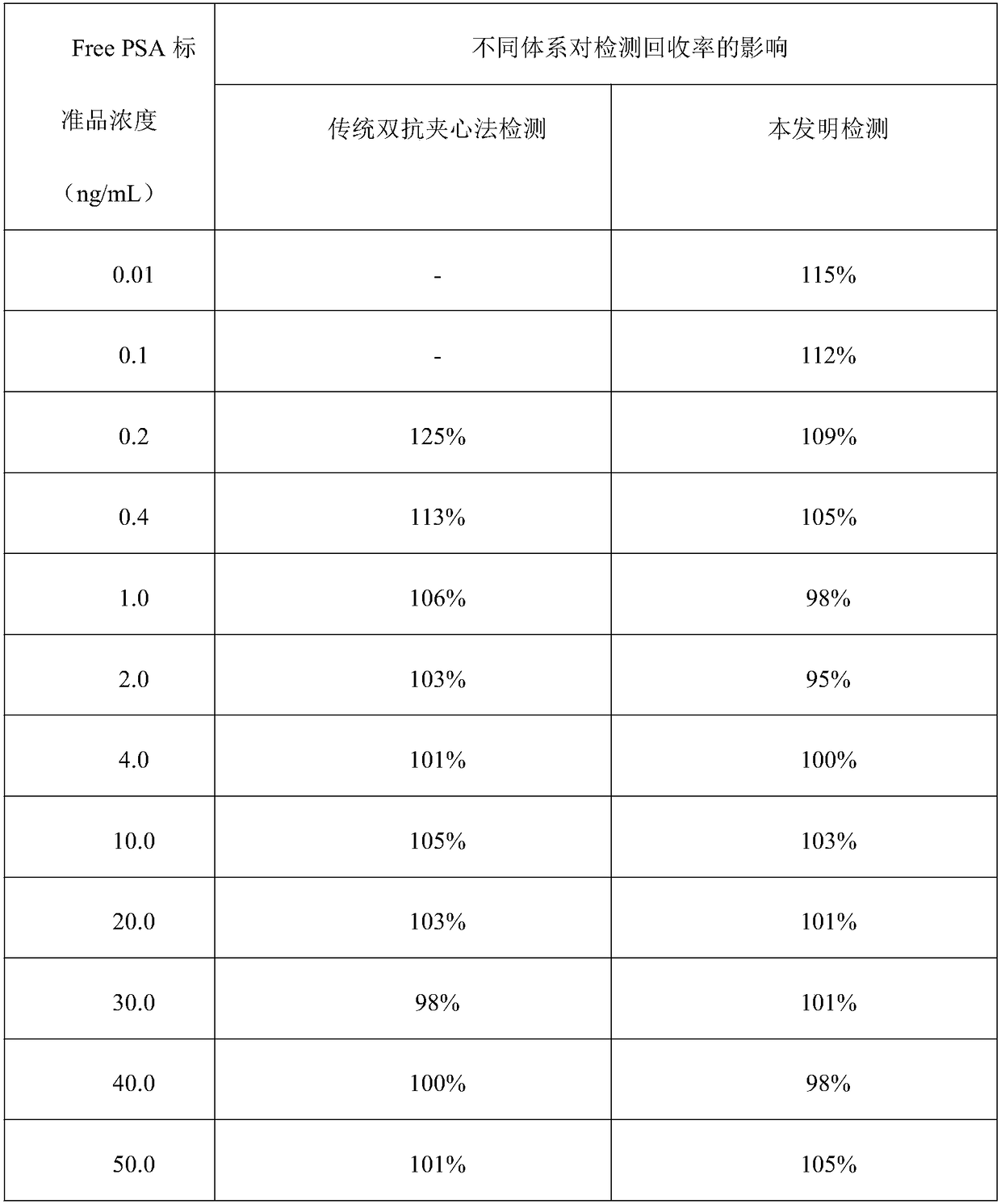
[0041] Embodiment 1: sandwich method comparative experiment (Free PSA)
[0042] 1. Solid phase carrier directly coated with antibody
[0043] 1. Take the magnetic bead stock solution, wash the magnetic beads twice with 50mM MES buffer solution, and then resuspend in the buffer solution. A certain concentration of EDC ready-made solution was added thereto, and activated at 37° C. for 0.5 hours. After activation, wash with MES buffer solution three times, and then resuspend in reaction buffer solution.
[0044] 2. Add the antibody used and react at 37°C for 3 hours. After the reaction is completed, wash with MES buffer solution three times, and finally resuspend in magnetic bead storage solution.
[0045] 2. Analysis steps
[0046] Concentration gradients were prepared with PBS buffer solution: 0.01, 0.1, 0.2, 0.4, 1.0, 2.0, 4.0, 10.0, 20.0, 30.0, 40.0, 50.0ng / mL Free PSA standard. In a cuvette, add 20 μL of magnetic beads coated with one strain of Free PSA monoclonal antibod...
Embodiment 2
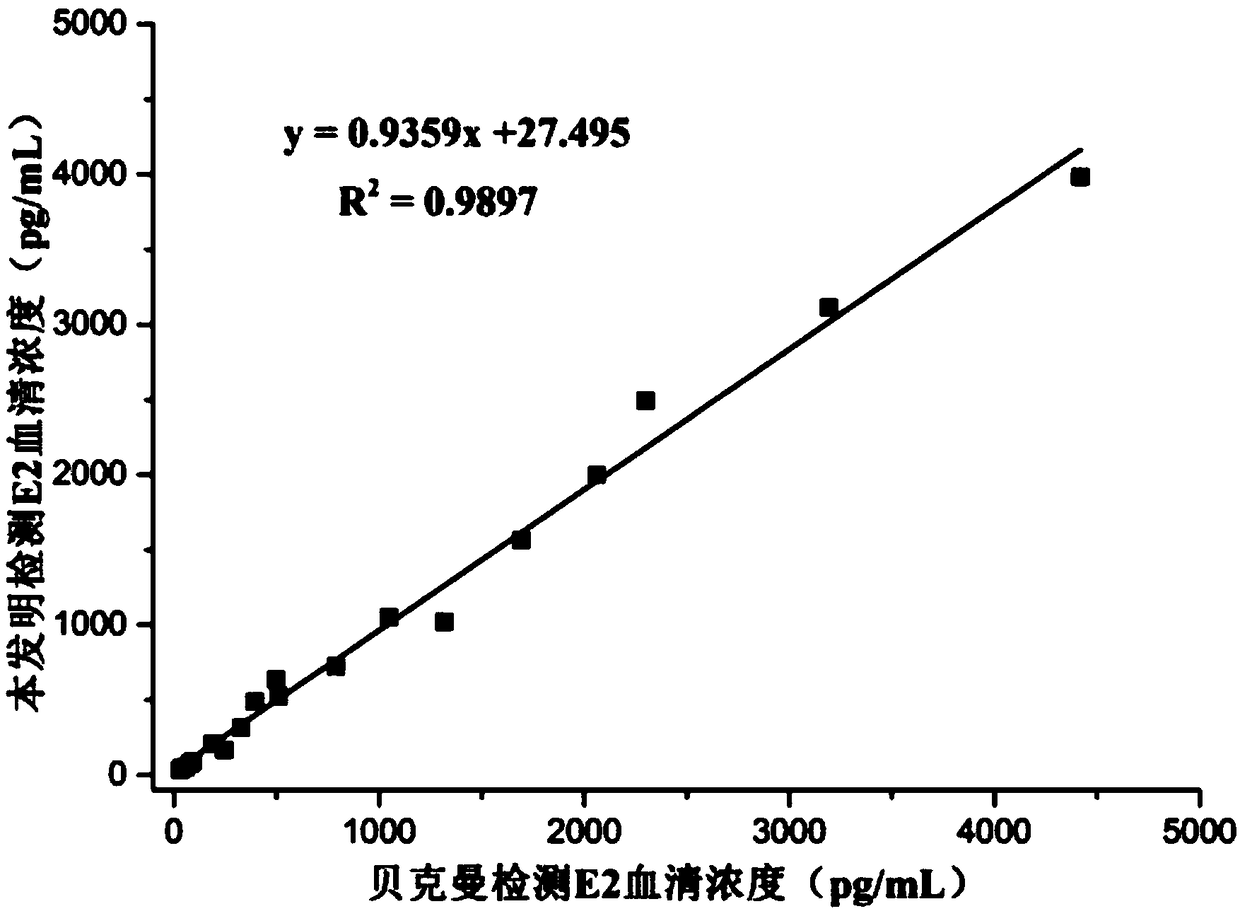
[0054] Embodiment 2: competition method and comparative test (E2)
[0055] 1. Solid phase carrier directly coated with antigen
[0056] 1. Take the original solution of magnetic beads, take the reaction buffer solution to wash the magnetic beads, and then resuspend in the buffer solution. A certain concentration of EDC ready-made solution was added thereto, and activated at 37° C. for 0.5 hours. After activation, wash three times with magnetic bead washing solution, and then resuspend in reaction buffer solution.
[0057] 2. Add the antigen used and react at 37°C for 3 hours. After the reaction is completed, wash with magnetic bead washing solution three times, and finally resuspend in magnetic bead storage solution.
[0058] 2. Analysis steps
[0059] Concentration gradients were prepared with PBS buffer solution: 10, 20, 40, 80, 160, 320, 640, 1200, 2500, 3000, 4000, 4800pg / mL E2 standard. Add 30 μL E2 standard, 20 μL E2 displacer, 50 μL biotin-labeled E2 monoclonal ant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com